Aging and DNA repair US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Aging and DNA repair. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Aging and DNA repair US Medical PG Question 1: A 5-month-old male infant from a consanguineous marriage presents with severe sunburns and freckling in sun exposed areas. The mother explains that the infant experiences these sunburns every time the infant goes outside despite applying copious amounts of sunscreen. Which of the following DNA repair mechanisms is defective in this child?
- A. Non-homologous end joining
- B. Homologous recombination
- C. Base excision repair
- D. Mismatch repair
- E. Nucleotide excision repair (Correct Answer)
Aging and DNA repair Explanation: ***Nucleotide excision repair***
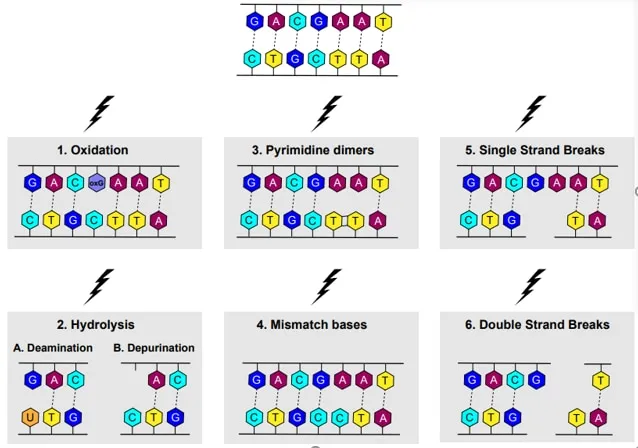
- The symptoms of **severe sunburns** and **freckling in sun-exposed areas** are classic manifestations of **Xeroderma Pigmentosum (XP)**.
- XP is caused by a defect in **nucleotide excision repair (NER)**, which is crucial for removing **UV-induced DNA damage**, such as **pyrimidine dimers**.
*Non-homologous end joining*
- This mechanism repairs **double-strand DNA breaks** by directly ligating the broken ends, often with some loss of genetic information.
- Defects in non-homologous end joining are associated with conditions like **immunodeficiency** and increased cancer risk, but not with UV sensitivity like XP.
*Homologous recombination*
- This high-fidelity repair pathway uses a **homologous DNA template** to accurately repair **double-strand breaks** and interstrand crosslinks.
- Impaired homologous recombination is linked to conditions like **Fanconi anemia** and increased risk of certain cancers, but not primarily to UV hypersensitivity.
*Base excision repair*
- **Base excision repair (BER)** is responsible for removing **damaged or modified bases** from DNA, such as oxidized or alkylated bases.
- Defects in BER can lead to increased spontaneous mutagenesis and cancer, but do not explain the specific sensitivity to UV light seen in this infant.
*Mismatch repair*
- **Mismatch repair (MMR)** corrects errors that occur during DNA replication, such as **base mismatches** or small insertions/deletions.
- Defective MMR is strongly associated with **hereditary nonpolyposis colorectal cancer (Lynch syndrome)**, but not with severe reactions to sun exposure.
Aging and DNA repair US Medical PG Question 2: An investigator is studying the normal process of shrinking of the thymus gland with increasing age in humans. Thymic size is found to gradually start decreasing during puberty. Which of the following enzymes is most likely involved in the process underlying the decline in thymus mass with aging?
- A. Lipase
- B. Collagenase
- C. Metalloproteinase
- D. Caspase (Correct Answer)
- E. NADPH oxidase
Aging and DNA repair Explanation: **Caspase**
- The shrinking of the thymus with age, known as **thymic involution**, is primarily driven by **apoptosis** (programmed cell death) of thymocytes.
- **Caspases** are a family of proteases that play a central role in initiating and executing apoptosis, making them the most likely enzymes involved in this process.
*Lipase*
- **Lipases** are enzymes that catalyze the hydrolysis of fats (lipids).
- While fat deposition occurs in the involuting thymus, lipases are not directly responsible for the **cell death** or tissue regression.
*Collagenase*
- **Collagenases** are enzymes that break down **collagen**, a major component of the extracellular matrix.
- While there may be some remodeling of the extracellular matrix during thymic involution, collagenases are not the primary drivers of **thymocyte apoptosis**.
*Metalloproteinase*
- **Metalloproteinases (MMPs)** are enzymes that break down various components of the extracellular matrix and are involved in tissue remodeling.
- While MMPs contribute to tissue restructuring, they are not the main enzymes responsible for the **programmed cell death** that underpins thymic involution.
*NADPH oxidase*
- **NADPH oxidase** is an enzyme complex that produces **reactive oxygen species (ROS)**, primarily for pathogen killing by phagocytes.
- While excessive ROS can induce cell death, **NADPH oxidase** is not the primary or direct mechanism responsible for the physiological apoptosis during thymic involution.
Aging and DNA repair US Medical PG Question 3: A 17-year-old patient presents to the emergency department with left wrist pain after falling off of his bike and landing on his left hand. On physical exam the thenar eminence is red, swollen, and tender to palpation, so a radiograph is ordered. The patient is worried because he learned in biology class that radiography can cause cancer through damaging DNA but the physician reassures him that radiographs give a very minor dose of radiation. What is the most common mechanism by which ionizing radiation damages DNA?
- A. Strand breakage (Correct Answer)
- B. Thymidine dimer formation
- C. Microsatellite instability
- D. Cyclobutane pyrimidine dimer formation
- E. Cytosine deamination
Aging and DNA repair Explanation: ***Strand breakage***
- Ionizing radiation, such as X-rays, directly or indirectly causes **breaks in the phosphodiester backbone** of DNA, resulting in single or double-strand breaks.
- **Double-strand breaks** are particularly dangerous as they are difficult to repair and can lead to chromosomal rearrangements and cell death or malignant transformation.
*Thymidine dimer formation*
- This is primarily caused by **ultraviolet (UV) radiation**, not ionizing radiation like X-rays.
- **UV radiation** causes covalent bonds between adjacent pyrimidine bases, particularly thymine, leading to the formation of thymine dimers.
*Microsatellite instability*
- This is a hallmark of defects in **DNA mismatch repair pathways**, often associated with hereditary disorders like Lynch syndrome or certain sporadic cancers.
- It involves changes in the length of **microsatellites** (short, repetitive DNA sequences) due to insertion or deletion errors, not direct radiation damage.
*Cyclobutane pyrimidine dimer formation*
- Similar to thymidine dimers, **cyclobutane pyrimidine dimers (CPDs)** are the most common photoproducts formed in DNA after exposure to **UV radiation**.
- These dimers distort the DNA helix and interfere with replication and transcription, but are not characteristic of ionizing radiation damage.
*Cytosine deamination*
- This is a spontaneous chemical reaction where a **cytosine base (C)** loses its amino group and is converted to **uracil (U)**.
- It is a common endogenous DNA lesion that can lead to C-to-T transition mutations if not repaired, but it is not directly induced by ionizing radiation.
Aging and DNA repair US Medical PG Question 4: While performing a Western blot, a graduate student spilled a small amount of the radiolabeled antibody on her left forearm. Although very little harm was done to the skin, the radiation did cause minor damage to the DNA of the exposed skin by severing covalent bonds between the nitrogenous bases and the deoxyribose sugar, leaving several apurinic/apyrimidinic sites. Damaged cells would most likely repair these sites by which of the following mechanisms?
- A. Nucleotide excision repair
- B. Nonhomologous end joining repair
- C. Homologous recombination
- D. Mismatch repair
- E. Base excision repair (Correct Answer)
Aging and DNA repair Explanation: **Base excision repair**
- This mechanism is specifically involved in correcting **single-base DNA damage** or **modified bases**, such as **apurinic/apyrimidinic (AP) sites**.
- It involves removing the damaged base by a **DNA glycosylase**, creating an AP site, which is then processed by an **AP endonuclease** to cleave the phosphodiester backbone, followed by DNA polymerase and ligase.
*Nucleotide excision repair*
- Primarily repairs **bulky DNA lesions**, such as **thymine dimers** caused by UV radiation, or damage from chemical adducts that distort the DNA helix.
- It involves excising a larger oligonucleotide containing the damage, not just a single base.
*Nonhomologous end joining repair*
- This pathway is used to repair **double-strand DNA breaks**, where both strands of the DNA molecule are broken.
- It is a "quick-and-dirty" repair mechanism that ligates the broken ends together, often leading to small insertions or deletions.
*Homologous recombination*
- A repair mechanism for **double-strand DNA breaks** that uses a homologous DNA template (e.g., sister chromatid) to accurately repair the break.
- This process is highly accurate but occurs only when a homologous template is available, typically during the S and G2 phases of the cell cycle.
*Mismatch repair*
- Corrects **base-pair mismatches** and **small insertions/deletions** that occur during DNA replication, which were not corrected by DNA polymerase proofreading.
- It targets newly synthesized DNA strands based on methylation patterns in the parental strand.
Aging and DNA repair US Medical PG Question 5: A 3-year-old is brought to the pediatrician by his mother because she is concerned about recent changes to his behavior. She states that he has seemed to regress in his motor development and has been having occasional brief episodes of uncontrollable shaking. During the subsequent work up, a muscle biopsy is obtained which demonstrates red ragged fibers and a presumptive diagnosis of a genetic disease made. The mother asks if her other son will be affected. What should be the physician's response?
- A. There is a 50% chance he will be affected
- B. There is a 100% chance he will be affected, and the severity will be the same
- C. There is a 25% chance he will be affected
- D. There is a 100% chance he will be affected, but the severity may be different (Correct Answer)
- E. He will be unaffected
Aging and DNA repair Explanation: ***There is a 100% chance he will be affected, but the severity may be different***
- The patient's symptoms (motor regression, seizures, red ragged fibers on muscle biopsy) are classic for a **mitochondrial disorder**, which are inherited via **maternal inheritance**.
- All children of an affected mother will inherit the affected mitochondria; however, the **heteroplasmy** (proportion of mutated mitochondria inherited) can vary, leading to different disease severities.
*There is a 50% chance he will be affected*
- This inheritance pattern is typical for **autosomal dominant** disorders, or occasionally X-linked disorders in males.
- Mitochondrial disorders do not follow autosomal dominant inheritance, as they are exclusively inherited from the mother.
*There is a 100% chance he will be affected, and the severity will be the same*
- While there is a 100% chance of inheriting the mutated mitochondria from an affected mother, the **phenotypic expression and severity can vary widely** due to heteroplasmy.
- The proportion of mutated mitochondria can differ in various tissues and between offspring, leading to variable clinical manifestations.
*There is a 25% chance he will be affected*
- This represents the risk of inheritance for an **autosomal recessive** disorder when both parents are carriers.
- Mitochondrial inheritance does not follow an autosomal recessive pattern.
*He will be unaffected*
- This would only be true if the mother's mitochondrial DNA were not affected or if the inheritance pattern allowed for some children to be completely spared, which is not the case for mitochondrial disorders.
- Since the mother is the carrier of the mitochondrial mutation, all her children will inherit the mutated mitochondria.
Aging and DNA repair US Medical PG Question 6: A 2-year-old boy from a rural community is brought to the pediatrician after his parents noticed a white reflection in both of his eyes in recent pictures. Physical examination reveals bilateral leukocoria, nystagmus, and inflammation. When asked about family history of malignancy, the father of the child reports losing a brother to an eye tumor when they were children. With this in mind, which of the following processes are affected in this patient?
- A. Base excision repair
- B. Regulation of the G1-S transition (Correct Answer)
- C. DNA mismatch repair
- D. Stem cell self-renewal
- E. Nucleotide excision repair
Aging and DNA repair Explanation: ***Regulation of the G1-S transition***
- This patient's symptoms (bilateral **leukocoria**, **nystagmus**, family history of eye tumor) are characteristic of **retinoblastoma**, which is often caused by a mutation in the **RB1 gene**.
- The **RB1 gene** product (retinoblastoma protein) is a key **tumor suppressor** that regulates the G1-S cell cycle transition, and its dysfunction leads to uncontrolled cell proliferation.
*Base excision repair*
- This process is primarily involved in repairing damaged bases in DNA, often due to oxidation or alkylation.
- Defects in base excision repair are typically associated with conditions such as **MUTYH-associated polyposis**, not retinoblastoma.
*DNA mismatch repair*
- This system corrects errors that occur during DNA replication, such as incorrect base pairings or small insertions/deletions.
- Impairment of mismatch repair is a hallmark of **Lynch syndrome** (hereditary nonpolyposis colorectal cancer), which does not present with retinoblastoma.
*Stem cell self-renewal*
- While uncontrolled self-renewal can contribute to cancer, retinoblastoma is specifically linked to defects in the **RB1 gene**, which is a cell cycle regulator, not directly a primary regulator of stem cell self-renewal itself.
- Loss of G1-S checkpoint control is a more direct and proximal cause of the tumor formation in retinoblastoma.
*Nucleotide excision repair*
- This pathway is responsible for repairing bulkier DNA lesions, such as those caused by UV radiation.
- Deficiencies in nucleotide excision repair lead to diseases like **xeroderma pigmentosum**, characterized by extreme sensitivity to sunlight and increased skin cancer risk, which is unrelated to the presented case.
Aging and DNA repair US Medical PG Question 7: An investigator studying DNA mutation mechanisms isolates single-stranded DNA from a recombinant bacteriophage and sequences it. The investigator then mixes it with a buffer solution and incubates the resulting mixture at 70°C for 16 hours. Subsequent DNA resequencing shows that 3.7 per 1,000 cytosine residues have mutated to uracil. Which of the following best describes the role of the enzyme that is responsible for the initial step in repairing these types of mutations in living cells?
- A. Connecting the phosphodiester backbone
- B. Cleavage of the phosphodiester bond 3' of damaged site
- C. Creation of abasic site (Correct Answer)
- D. Release of the damaged nucleotide
- E. Addition of free nucleotides to 3' end
Aging and DNA repair Explanation: ***Creation of abasic site***
- The mutation of **cytosine to uracil** is an example of **deamination**, which is repaired by the **base excision repair (BER)** pathway.
- The initial step in BER involves **DNA glycosylase**, which *removes* the damaged base (uracil) from the sugar-phosphate backbone by hydrolyzing the **N-glycosidic bond**, creating an **abasic site**.
*Connecting the phosphodiester backbone*
- This is the function of **DNA ligase**, which acts at the *final step* of DNA repair pathways to seal the nicks in the backbone.
- It does not initiate the repair process for deaminated bases.
*Cleavage of the phosphodiester bond 3' of damaged site*
- This is typically performed by an **AP endonuclease (APE1)** after the abasic site has been created.
- It is a *subsequent step* in BER, not the initial one for removing the damaged base itself.
*Release of the damaged nucleotide*
- While the damaged base is eventually *released*, the initial enzyme (DNA glycosylase) specifically removes the **base**, leaving the sugar and phosphate intact.
- The entire nucleotide (base, sugar, and phosphate) is typically removed later by an **AP lyase** or APE1, after the initial glycosylase action.
*Addition of free nucleotides to 3' end*
- This is the function of **DNA polymerase**, which fills in the gap after the damaged nucleotide and surrounding region have been excised.
- This occurs *after* the initial recognition and removal of the damaged base, not as the primary repair step.
Aging and DNA repair US Medical PG Question 8: A 54-year-old woman with breast cancer comes to the physician because of redness and pain in the right breast. She has been undergoing ionizing radiation therapy daily for the past 2 weeks as adjuvant treatment for her breast cancer. Physical examination shows erythema, edema, and superficial desquamation of the skin along the right breast at the site of radiation. Sensation to light touch is intact. Which of the following is the primary mechanism of DNA repair responsible for preventing radiation-induced damage to neighboring neurons?
- A. Homology-directed repair
- B. Base excision repair
- C. Nonhomologous end joining repair (Correct Answer)
- D. DNA mismatch repair
- E. Nucleotide excision repair
Aging and DNA repair Explanation: ***Nonhomologous end joining repair***
- This pathway is crucial for repairing **double-strand DNA breaks**, which are a major form of damage caused by **ionizing radiation**.
- It directly ligates the broken DNA ends without requiring a homologous template, making it an efficient but potentially error-prone repair mechanism.
*Homology-directed repair*
- This pathway is also used to repair **double-strand DNA breaks** but requires a **homologous DNA template** (usually a sister chromatid) for accurate repair.
- While highly accurate, it is typically active during the S and G2 phases of the cell cycle and is generally slower and less dominant than NHEJ for immediate radiation-induced damage in non-dividing cells like neurons.
*Base excision repair*
- This mechanism primarily corrects damage to individual DNA bases, such as **oxidative damage**, alkylation, or deamination.
- It is not the primary mechanism for repairing the **double-strand breaks** induced by ionizing radiation.
*DNA mismatch repair*
- This pathway corrects errors that arise during **DNA replication**, specifically mismatched base pairs or small insertions/deletions.
- It is not involved in repairing radiation-induced DNA damage like **double-strand breaks**.
*Nucleotide excision repair*
- This pathway repairs bulky DNA lesions, such as those caused by **UV radiation** (e.g., pyrimidine dimers) or chemical mutagens.
- It removes a segment of DNA containing the damage but is not the primary repair mechanism for **double-strand breaks** caused by ionizing radiation.
Aging and DNA repair US Medical PG Question 9: A 3-year-old male child is found to have a disease involving DNA repair. Specifically, he is found to have a defect in the endonucleases involved in the nucleotide excision repair of pyrimidine dimers. Which of the following is a unique late-stage complication of this child's disease?
- A. Telangiectasia
- B. Colorectal cancer
- C. Malignant melanoma (Correct Answer)
- D. Lymphomas
- E. Endometrial cancer
Aging and DNA repair Explanation: **Malignant melanoma**
- The described condition is **xeroderma pigmentosum**, an autosomal recessive disorder characterized by a defect in **nucleotide excision repair (NER)**, specifically the inability to remove **pyrimidine dimers** caused by **UV radiation**.
- This severely impaired DNA repair leads to an extreme predisposition to **UV-induced skin cancers**, including basal cell carcinomas, squamous cell carcinomas, and, most aggressively, **malignant melanoma**, which is a unique and life-threatening late-stage complication.
*Telangiectasia*
- **Telangiectasias** are dilated small blood vessels that appear on the skin or mucous membranes and can be associated with various conditions.
- While skin abnormalities are prevalent in xeroderma pigmentosum due to sun damage, **melanoma** is a more specific and severe late-stage complication directly resulting from the DNA repair defect.
*Colorectal cancer*
- **Colorectal cancer** is typically associated with other DNA repair defects, such as those in the **mismatch repair system**, as seen in conditions like **Lynch syndrome**.
- It is not a primary or most significant late-stage complication of xeroderma pigmentosum, which is primarily characterized by skin cancers.
*Lymphomas*
- **Lymphomas** are cancers of the lymphatic system, often linked to immune deficiencies or specific genetic translocations.
- While individuals with genetic syndromes can have increased cancer risks, **lymphoma** is not the hallmark late-stage complication of xeroderma pigmentosum; skin cancers are the predominant concern.
*Endometrial cancer*
- **Endometrial cancer** is a gynecological cancer often associated with hormonal factors or genetic predispositions like Lynch syndrome, which involves mismatch repair defects.
- This type of cancer is not a characteristic or unique late-stage complication of xeroderma pigmentosum, whose pathology is centered on **UV-induced DNA damage** and subsequent skin malignancies.
Aging and DNA repair US Medical PG Question 10: As part of a clinical research study, the characteristics of neoplastic and normal cells are being analyzed in culture. It is observed that neoplastic cell division is aided by an enzyme which repairs progressive chromosomal shortening, which is not the case in normal cells. Due to the lack of chromosomal shortening, these neoplastic cells divide more rapidly than the normal cells. Which of the following enzymes is most likely involved?
- A. Topoisomerase
- B. DNA polymerase
- C. Reverse transcriptase
- D. Protein kinase
- E. Telomerase (Correct Answer)
Aging and DNA repair Explanation: ***Telomerase***
- **Telomerase** is an enzyme that adds repetitive nucleotide sequences (telomeres) to the ends of chromosomes, counteracting their progressive shortening during DNA replication. This activity is crucial for the continuous division of neoplastic cells.
- In normal somatic cells, **telomerase activity is typically low or absent**, leading to telomere shortening with each division, eventually triggering cellular senescence or apoptosis. The presence of telomerase in neoplastic cells allows them to bypass these natural limits on proliferation.
*Topoisomerase*
- **Topoisomerases** are enzymes that regulate the supercoiling of DNA by breaking and rejoining DNA strands, which is essential during replication and transcription to relieve torsional stress.
- They do not directly repair chromosomal shortening but rather manage the topological state of DNA.
*DNA polymerase*
- **DNA polymerase** is primarily responsible for synthesizing new DNA strands by adding nucleotides, thereby elongating the DNA molecule during replication and DNA repair processes.
- While essential for DNA replication, it cannot fully replicate the very ends of linear chromosomes, leading to the **end-replication problem** and telomere shortening.
*Reverse transcriptase*
- **Reverse transcriptase** is an enzyme that synthesizes DNA from an RNA template, a process central to retroviruses and some eukaryotic elements like retrotransposons.
- Although telomerase itself is a specialized reverse transcriptase (using an RNA template to synthesize DNA telomeres), the general term "reverse transcriptase" does not specifically refer to the enzyme that repairs chromosomal shortening in the context of cell division.
*Protein kinase*
- **Protein kinases** are enzymes that add phosphate groups to proteins, a process known as phosphorylation. This modification can alter protein activity, localization, or stability, playing a critical role in signal transduction pathways.
- They are involved in regulating various cellular processes, including cell growth and division, but do not directly repair chromosomal shortening.
More Aging and DNA repair US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.