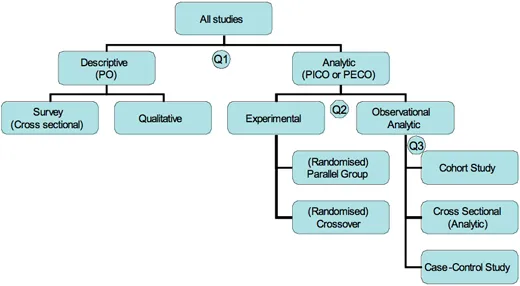
Survey design US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Survey design. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Survey design US Medical PG Question 1: A researcher is studying whether a new knee implant is better than existing alternatives in terms of pain after knee replacement. She designs the study so that it includes all the surgeries performed at a certain hospital. Interestingly, she notices that patients who underwent surgeries on Mondays and Thursdays reported much better pain outcomes on a survey compared with those who underwent the same surgeries from the same surgeons on Tuesdays and Fridays. Upon performing further analysis, she discovers that one of the staff members who works on Mondays and Thursdays is aware of the study and tells all the patients about how wonderful the new implant is. Which of the following forms of bias does this most likely represent?
- A. Hawthorne effect
- B. Pygmalion effect (Correct Answer)
- C. Attrition bias
- D. Golem effect
Survey design Explanation: ***Pygmalion effect***
- This bias occurs when higher expectations lead to an increase in performance. In this scenario, the staff member's positive reinforcement about the new implant likely instilled **higher patient expectations**, leading to better reported pain outcomes.
- The patients' belief in the implant's superiority, influenced by the staff member, acted as a **self-fulfilling prophecy**, improving their subjective pain experience.
*Hawthorne effect*
- This effect describes how individuals modify an aspect of their behavior in response to their awareness of being observed. While patients were part of a study, their improved outcomes were specifically linked to a staff member's verbal influence, not solely the act of observation.
- The improved pain outcomes stem from the **expectations created by the staff member's praise**, rather than a general awareness of being studied.
*Attrition bias*
- Attrition bias refers to systematic differences between groups in the loss of participants from a study.
- This scenario describes differences in patient outcomes based on staff influence during the study, not due to **patients dropping out differentially** between groups.
*Golem effect*
- The Golem effect is the opposite of the Pygmalion effect, where lower expectations placed upon individuals lead to poorer performance from them.
- In this case, the staff member's influence created **high expectations and positive outcomes**, not negative expectations leading to worse outcomes.
Survey design US Medical PG Question 2: A researcher is trying to determine whether a newly discovered substance X can be useful in promoting wound healing after surgery. She conducts this study by enrolling the next 100 patients that will be undergoing this surgery and separating them into 2 groups. She decides which patient will be in which group by using a random number generator. Subsequently, she prepares 1 set of syringes with the novel substance X and 1 set of syringes with a saline control. Both of these sets of syringes are unlabeled and the substances inside cannot be distinguished. She gives the surgeon performing the surgery 1 of the syringes and does not inform him nor the patient which syringe was used. After the study is complete, she analyzes all the data that was collected and performs statistical analysis. This study most likely provides which level of evidence for use of substance X?
- A. Level 3
- B. Level 1 (Correct Answer)
- C. Level 4
- D. Level 5
- E. Level 2
Survey design Explanation: ***Level 1***
- The study design described is a **randomized controlled trial (RCT)**, which is considered the **highest level of evidence (Level 1)** in the hierarchy of medical evidence.
- Key features like **randomization**, **control group**, and **blinding (double-blind)** help minimize bias and strengthen the validity of the findings.
*Level 2*
- Level 2 evidence typically comprises **well-designed controlled trials without randomization** (non-randomized controlled trials) or **high-quality cohort studies**.
- While strong, they do not possess the same level of internal validity as randomized controlled trials.
*Level 3*
- Level 3 evidence typically includes **case-control studies** or **cohort studies**, which are observational designs and carry a higher risk of bias compared to RCTs.
- These studies generally do not involve randomization or intervention assignment by the researchers.
*Level 4*
- Level 4 evidence is usually derived from **case series** or **poor quality cohort and case-control studies**.
- These studies provide descriptive information or investigate associations without strong control for confounding factors.
*Level 5*
- Level 5 evidence is the **lowest level of evidence**, consisting of **expert opinion** or **animal research/bench research**.
- This level lacks human clinical data or systematic investigative rigor needed for higher evidence levels.
Survey design US Medical PG Question 3: You are reading through a recent article that reports significant decreases in all-cause mortality for patients with malignant melanoma following treatment with a novel biological infusion. Which of the following choices refers to the probability that a study will find a statistically significant difference when one truly does exist?
- A. Type II error
- B. Type I error
- C. Confidence interval
- D. p-value
- E. Power (Correct Answer)
Survey design Explanation: ***Power***
- **Power** is the probability that a study will correctly reject the null hypothesis when it is, in fact, false (i.e., will find a statistically significant difference when one truly exists).
- A study with high power minimizes the risk of a **Type II error** (failing to detect a real effect).
*Type II error*
- A **Type II error** (or **beta error**) occurs when a study fails to reject a false null hypothesis, meaning it concludes there is no significant difference when one actually exists.
- This is the **opposite** of what the question describes, which asks for the probability of *finding* a difference.
*Type I error*
- A **Type I error** (or **alpha error**) occurs when a study incorrectly rejects a true null hypothesis, concluding there is a significant difference when one does not actually exist.
- This relates to the **p-value** and the level of statistical significance (e.g., p < 0.05).
*Confidence interval*
- A **confidence interval** provides a range of values within which the true population parameter is likely to lie with a certain degree of confidence (e.g., 95%).
- It does not directly represent the probability of finding a statistically significant difference when one truly exists.
*p-value*
- The **p-value** is the probability of observing data as extreme as, or more extreme than, that obtained in the study, assuming the null hypothesis is true.
- It is used to determine statistical significance, but it is not the probability of detecting a true effect.
Survey design US Medical PG Question 4: The APPLE study investigators are currently preparing for a 30-year follow-up evaluation. They are curious about the number of participants who will partake in follow-up interviews. The investigators noted that of the 83 participants who participated in the APPLE study's 20-year follow-up, 62 were in the treatment group and 21 were in the control group. Given the unequal distribution of participants between groups at follow-up, this finding raises concerns for which of the following?
- A. Volunteer bias
- B. Reporting bias
- C. Inadequate sample size
- D. Attrition bias (Correct Answer)
- E. Lead-time bias
Survey design Explanation: ***Attrition bias***
- **Attrition bias** occurs when participants drop out of a study, especially if the dropout rate differs between the intervention and control groups, which can lead to a **skewed comparison** of outcomes.
- The unequal distribution of participants (62 vs. 21) between the treatment and control groups at the 20-year follow-up suggests that a disproportionate number of participants may have dropped out of one group, thus leading to attrition bias.
*Volunteer bias*
- **Volunteer bias** occurs when individuals who volunteer for a study differ significantly from the general population or those who decline to participate, potentially affecting the study's **generalizability**.
- This scenario describes differences in retention *after* initial participation, not differences in initial willingness to join.
*Reporting bias*
- **Reporting bias** refers to the selective reporting of study findings, where positive or statistically significant results are more likely to be published or emphasized than negative or non-significant ones, which can distort the overall evidence base.
- This bias relates to how results are disseminated, not to differential dropout rates or participant retention in a study.
*Inadequate sample size*
- **Inadequate sample size** means that the number of participants in a study is too small to detect a statistically significant effect if one truly exists, leading to a lack of **statistical power**.
- While the overall number of participants at follow-up might be small, the primary concern here is the *unequal distribution* between groups, indicating a problem with participant retention rather than just a low total count.
*Lead-time bias*
- **Lead-time bias** occurs when early detection of a disease (e.g., through screening) makes survival appear longer than it actually is, without necessarily prolonging the patient's life, by advancing the **point of diagnosis**.
- This bias is relevant to screening programs and disease detection, not to the differential dropout rates observed in a longitudinal study.
Survey design US Medical PG Question 5: Study X examined the relationship between coffee consumption and lung cancer. The authors of Study X retrospectively reviewed patients' reported coffee consumption and found that drinking greater than 6 cups of coffee per day was associated with an increased risk of developing lung cancer. However, Study X was criticized by the authors of Study Y. Study Y showed that increased coffee consumption was associated with smoking. What type of bias affected Study X, and what study design is geared to reduce the chance of that bias?
- A. Observer bias; double blind analysis
- B. Selection bias; randomization
- C. Lead time bias; placebo
- D. Measurement bias; blinding
- E. Confounding; randomization (Correct Answer)
Survey design Explanation: ***Confounding; randomization***
- Study Y suggests that **smoking** is a **confounding variable** because it is associated with both increased coffee consumption (exposure) and increased risk of lung cancer (outcome), distorting the apparent relationship between coffee and lung cancer.
- **Randomization** in experimental studies (such as randomized controlled trials) helps reduce confounding by ensuring that known and unknown confounding factors are evenly distributed among study groups.
- In observational studies where randomization is not possible, confounding can be addressed through **stratification**, **matching**, or **multivariable adjustment** during analysis.
*Observer bias; double blind analysis*
- **Observer bias** occurs when researchers' beliefs or expectations influence the study outcome, which is not the primary issue described here regarding the relationship between coffee, smoking, and lung cancer.
- **Double-blind analysis** is a method to mitigate observer bias by ensuring neither participants nor researchers know who is in the control or experimental groups.
*Selection bias; randomization*
- **Selection bias** happens when the study population is not representative of the target population, leading to inaccurate results, which is not directly indicated by the interaction between coffee and smoking.
- While **randomization** is used to reduce selection bias by creating comparable groups, the core problem identified in Study X is confounding, not flawed participant selection.
*Lead time bias; placebo*
- **Lead time bias** occurs in screening programs when early detection without improved outcomes makes survival appear longer, an issue unrelated to the described association between coffee, smoking, and lung cancer.
- A **placebo** is an inactive treatment used in clinical trials to control for psychological effects, and its relevance here is limited to treatment intervention studies.
*Measurement bias; blinding*
- **Measurement bias** arises from systematic errors in data collection, such as inaccurate patient reporting of coffee consumption, but the main criticism from Study Y points to a third variable (smoking) affecting the association, not just flawed measurement.
- **Blinding** helps reduce measurement bias by preventing participants or researchers from knowing group assignments, thus minimizing conscious or unconscious influences on data collection.
Survey design US Medical PG Question 6: A study is conducted in a hospital to estimate the prevalence of handwashing among healthcare workers. All of the hospital staff members are informed that the study is being conducted for 1 month, and the study method will be a passive observation of their daily routine at the hospital. A total of 89 medical staff members give their consent for the study, and they are followed for a month. This study could most likely suffer from which of the following biases?
- A. Attrition bias
- B. Hawthorne effect (Correct Answer)
- C. Confounding bias
- D. Berksonian bias
- E. Observer-expectancy bias
Survey design Explanation: ***Hawthorne effect***
- This bias occurs when individuals modify their behavior in response to being **observed** or knowing they are part of a study. In this scenario, healthcare workers, knowing they are being observed for handwashing, are likely to wash their hands more frequently than usual.
- The intent of the study is to estimate the **prevalence** of handwashing; however, the observed rates will be artificially inflated due to the subjects' awareness of being studied, leading to an inaccurate estimate.
*Attrition bias*
- **Attrition bias** arises when there is **differential loss to follow-up** between study groups, which can lead to biased results.
- This study design involves observing a defined group for a month, but there's no indication of loss of participants or differential dropout from specific intervention or control groups.
*Confounding bias*
- **Confounding bias** occurs when an unmeasured or uncontrolled factor (a **confounder**) is associated with both the exposure and the outcome, distorting the true association.
- While confounding is a common bias in observational studies, the primary issue described here is the direct impact of observation on behavior, not an unmeasured external variable influencing both the behavior and its measurement.
*Berksonian bias*
- **Berksonian bias** (or admission rate bias) is a type of selection bias that occurs in case-control studies when hospital-based controls or cases are used, and the probability of being admitted to the hospital is influenced by both the exposure and the disease itself.
- This study is a **prevalence study** involving direct observation of healthcare workers, not a case-control study, making Berksonian bias irrelevant.
*Observer-expectancy bias*
- **Observer-expectancy bias** occurs when the **researcher's expectations** or beliefs influence their observations or interpretation of data.
- The scenario describes the participants (healthcare workers) changing their behavior due to being observed, not the observer's expectations influencing the recorded data, which would be the **Hawthorne effect**.
Survey design US Medical PG Question 7: A 28-year-old male presents to his primary care physician with complaints of intermittent abdominal pain and alternating bouts of constipation and diarrhea. His medical chart is not significant for any past medical problems or prior surgeries. He is not prescribed any current medications. Which of the following questions would be the most useful next question in eliciting further history from this patient?
- A. "Does the diarrhea typically precede the constipation, or vice-versa?"
- B. "Is the diarrhea foul-smelling?"
- C. "Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life"
- D. "Are the symptoms worse in the morning or at night?"
- E. "Can you tell me more about the symptoms you have been experiencing?" (Correct Answer)
Survey design Explanation: ***Can you tell me more about the symptoms you have been experiencing?***
- This **open-ended question** encourages the patient to provide a **comprehensive narrative** of their symptoms, including details about onset, frequency, duration, alleviating/aggravating factors, and associated symptoms, which is crucial for diagnosis.
- In a patient presenting with vague, intermittent symptoms like alternating constipation and diarrhea, allowing them to elaborate freely can reveal important clues that might not be captured by more targeted questions.
*Does the diarrhea typically precede the constipation, or vice-versa?*
- While knowing the sequence of symptoms can be helpful in understanding the **pattern of bowel dysfunction**, it is a very specific question that might overlook other important aspects of the patient's experience.
- It prematurely narrows the focus without first obtaining a broad understanding of the patient's overall symptomatic picture.
*Is the diarrhea foul-smelling?*
- Foul-smelling diarrhea can indicate **malabsorption** or **bacterial overgrowth**, which are important to consider in some gastrointestinal conditions.
- However, this is a **specific symptom inquiry** that should follow a more general exploration of the patient's symptoms, as it may not be relevant if other crucial details are missed.
*Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life*
- Quantifying pain intensity is useful for assessing the **severity of discomfort** and monitoring changes over time.
- However, for a patient with intermittent rather than acute, severe pain, understanding the **character, location, and triggers** of the pain is often more diagnostically valuable than just a numerical rating initially.
*Are the symptoms worse in the morning or at night?*
- Diurnal variation can be relevant in certain conditions, such as inflammatory bowel diseases where nocturnal symptoms might be more concerning, or functional disorders whose symptoms might be stress-related.
- This is another **specific question** that should come after gathering a more complete initial picture of the patient's symptoms to ensure no key information is overlooked.
Survey design US Medical PG Question 8: A research team has data from three completed studies on statin use and Alzheimer's disease: Study A (case-control, OR=0.6, n=500), Study B (retrospective cohort, RR=0.7, n=10,000), and Study C (RCT with cognitive decline as secondary endpoint, RR=0.9, n=2,000). The case-control study used prevalent cases, the cohort study had significant loss to follow-up in the unexposed group, and the RCT was underpowered for cognitive outcomes. Synthesize the evidence to determine the most reliable conclusion about the association.
- A. The RCT provides the strongest evidence despite being underpowered
- B. The retrospective cohort study offers the best balance of validity and precision (Correct Answer)
- C. Case-control study is most reliable due to efficient rare outcome assessment
- D. Evidence is contradictory and no conclusion can be drawn
- E. Meta-analysis of all three studies provides the most accurate estimate
Survey design Explanation: ***The retrospective cohort study offers the best balance of validity and precision***
- Despite **differential loss to follow-up** in the unexposed, the very large **sample size (n=10,000)** provides high **statistical power** and precision compared to the other studies.
- Cohort studies establish a **temporal sequence** (statin use before disease onset), which is superior to cross-sectional or prevalent case-control designs for causal inference.
*The RCT provides the strongest evidence despite being underpowered*
- While **Randomized Controlled Trials (RCTs)** are higher in the evidence hierarchy, an **underpowered** study lacks the precision to detect a true effect, increasing the risk of a **Type II error**.
- Since cognitive decline was only a **secondary endpoint**, the study may not have been designed or followed long enough to assess Alzheimer's disease progression accurately.
*Case-control study is most reliable due to efficient rare outcome assessment*
- The use of **prevalent cases** instead of incident cases introduces **Neyman bias (survival bias)**, as it only includes patients who survived long enough to be studied.
- Case-control studies are prone to **recall bias** and cannot definitively prove that statin use preceded the onset of cognitive decline.
*Evidence is contradictory and no conclusion can be drawn*
- While the results vary, researchers can still synthesize evidence by weighing studies based on their **methodological quality**, **sample size**, and **bias profile**.
- A conclusion can be drawn by acknowledging the **conservative estimate** (bias toward the null) in the cohort study which still suggested a protective effect.
*Meta-analysis of all three studies provides the most accurate estimate*
- **Meta-analysis** of studies with fundamentally different designs (RCT, Cohort, Case-Control) and significant **methodological limitations** can lead to inaccurate pooled results ("garbage in, garbage out").
- Combining studies with different measures of association (OR vs RR) and distinct biases like **survival bias** and **differential attrition** increases **heterogeneity**.
Survey design US Medical PG Question 9: A public health department needs to determine whether a cluster of birth defects in a county is associated with industrial pollution. They have limited resources, the suspected exposure occurred 3-5 years ago, and the outcome is rare (15 cases identified). Multiple potential confounders exist including maternal age, socioeconomic status, and prenatal care access. The community demands rapid answers. Evaluate the most appropriate initial study design considering feasibility, ethics, and scientific validity.
- A. Prospective cohort study of pregnant women with exposure monitoring
- B. Randomized controlled trial comparing exposed and unexposed areas
- C. Case-control study with multiple control groups and confounder adjustment (Correct Answer)
- D. Ecologic study comparing county-level pollution and birth defect rates
- E. Cross-sectional survey of current pollution levels and birth outcomes
Survey design Explanation: ***Case-control study with multiple control groups and confounder adjustment***
- A **case-control study** is the gold standard for investigating **rare outcomes** (only 15 cases identified) because it identifies subjects based on disease status rather than waiting for it to develop.
- This design is highly efficient for **retrospective exposures** that occurred years ago, allows for **rapid results** with limited resources, and can adjust for multiple **confounders** like maternal age and SES through statistical modeling.
*Prospective cohort study of pregnant women with exposure monitoring*
- This design is inappropriate for **rare outcomes** because it would require an massive sample size and many years of follow-up before seeing enough cases to be statistically significant.
- It cannot address a **past exposure** (3-5 years ago) as it follows subjects forward in time from the point of current exposure.
*Randomized controlled trial comparing exposed and unexposed areas*
- It is fundamentally **unethical** to intentionally expose human populations to potentially harmful industrial pollutants in an experimental setting.
- RCTs are used for **interventions** (like new drugs) rather than investigating the etiology of environmental health hazards.
*Ecologic study comparing county-level pollution and birth defect rates*
- This design is prone to the **ecologic fallacy**, where associations found at the population level may not hold true for individuals within that population.
- It cannot adjust for **individual-level confounders** such as personal prenatal care access or specific maternal age, making the results scientifically weaker for the community's needs.
*Cross-sectional survey of current pollution levels and birth outcomes*
- This design suffers from **temporal ambiguity**, as it measures exposure and outcome simultaneously, failing to confirm that pollution Exposure preceded the birth defects.
- Current pollution levels may not accurately reflect the **historical exposure** that occurred during the critical window of embryogenesis 3-5 years ago.
Survey design US Medical PG Question 10: A pharmaceutical company wants to evaluate a new anticoagulant's effectiveness in preventing stroke in atrial fibrillation patients. They have limited funding and need results within 2 years. The drug has promising phase 2 data. Concurrent medications and comorbidities vary widely in the target population. The company must choose between a pragmatic trial in 50 community hospitals or an explanatory trial at 3 academic centers with strict protocols. Evaluate which design best serves both scientific and practical objectives.
- A. Cluster randomized trial across academic and community sites
- B. Explanatory trial provides definitive efficacy data with maximum internal validity
- C. Pragmatic trial in community settings with broad inclusion criteria (Correct Answer)
- D. Sequential design starting with explanatory trial then pragmatic trial
- E. Adaptive trial design with interim efficacy monitoring
Survey design Explanation: ***Pragmatic trial in community settings with broad inclusion criteria***
- A **pragmatic trial** evaluates **effectiveness** in real-world clinical practice, making it ideal for a target population with diverse comorbidities and concurrent medications.
- It offers higher **external validity** (generalizability) and is often more feasible and cost-effective within a short timeline compared to highly controlled designs.
*Cluster randomized trial across academic and community sites*
- While it group-randomizes sites, this design is more complex to coordinate and may not be necessary if the goal is individual-level **stroke prevention** results.
- It does not specifically address the need for **broad inclusion criteria** or the limited budget as effectively as a standard pragmatic design.
*Explanatory trial provides definitive efficacy data with maximum internal validity*
- **Explanatory trials** test **efficacy** under ideal, highly controlled conditions, which may not reflect the actual benefit in the general population with comorbidities.
- Strict protocols at only 3 centers lead to low **generalizability** and slower recruitment, potentially exceeding the 2-year deadline.
*Sequential design starting with explanatory trial then pragmatic trial*
- A **sequential design** would require significantly more time and **funding** than the current two-year budget constraints allow.
- Since the drug already has promising **Phase 2 data**, moving directly to an effectiveness-focused pragmatic trial is a more strategic use of resources.
*Adaptive trial design with interim efficacy monitoring*
- **Adaptive designs** allow for modifications based on interim data but are statistically complex and often more **expensive** to manage.
- While scientifically rigorous, it does not prioritize the need for **real-world effectiveness** data in community settings requested by the scenario.
More Survey design US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.