Randomized controlled trials US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Randomized controlled trials. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
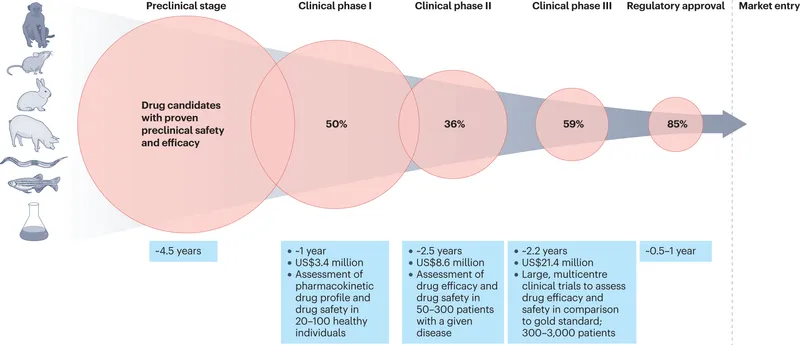
Randomized controlled trials US Medical PG Question 1: A 21-year-old man presents to the office for a follow-up visit. He was recently diagnosed with type 1 diabetes mellitus after being hospitalized for diabetic ketoacidosis following a respiratory infection. He is here today to discuss treatment options available for his condition. The doctor mentions a recent study in which researchers have developed a new version of the insulin pump that appears efficacious in type 1 diabetics. They are currently comparing it to insulin injection therapy. This new pump is not yet available, but it looks very promising. At what stage of clinical trials is this current treatment most likely at?
- A. Phase 0
- B. Phase 2
- C. Phase 3 (Correct Answer)
- D. Phase 1
- E. Phase 4
Randomized controlled trials Explanation: ***Phase 3***
- **Phase 3 trials** involve large-scale studies comparing the new treatment to standard therapy or placebo, often across multiple centers.
- The scenario describes a "new version of the insulin pump" being compared to "insulin injection therapy," indicating a definitive comparison for efficacy and safety against existing treatments.
*Phase 0*
- **Phase 0 trials** are exploratory, small-scale studies (10-15 subjects) using micro-doses to gather preliminary data on pharmacodynamics and pharmacokinetics, not efficacy comparisons.
- They are typically conducted very early in drug development, examining if the drug behaves as expected in humans.
*Phase 2*
- **Phase 2 trials** evaluate the efficacy and further assess safety of a new treatment in a larger group of patients (tens to hundreds).
- While they assess efficacy, they usually don't involve direct comparison with an established standard therapy on the scale implied by the question, which is typically reserved for Phase 3.
*Phase 1*
- **Phase 1 trials** primarily focus on safety, dosage, and side effects in a small group of healthy volunteers or patients with the condition (20-100 subjects).
- These trials are not designed to assess a treatment's efficacy against an existing therapy.
*Phase 4*
- **Phase 4 trials** occur after a drug or device has been approved and marketed, focusing on long-term safety, effectiveness in diverse populations, and new indications.
- The described pump "is not yet available," indicating it has not reached the market and thus is not in Phase 4.
Randomized controlled trials US Medical PG Question 2: A group of investigators seeks to compare the non-inferiority of a new angiotensin receptor blocker, salisartan, with losartan for reduction of blood pressure. 2,000 patients newly diagnosed with hypertension are recruited for the trial; the first 1,000 recruited patients are administered losartan, and the other half are administered salisartan. Patients with a baseline systolic blood pressure less than 100 mmHg are excluded from the study. Blood pressure is measured every week for four weeks, with the primary outcome being a reduction in systolic blood pressure by salisartan within 10% of that of the control. Secondary outcomes include incidence of subjective improvement in symptoms, improvement of ejection fraction, and incidence of cough. 500 patients withdraw from the study due to symptomatic side effects. In an intention-to-treat analysis, salisartan is deemed to be non-inferior to losartan for the primary outcome but inferior for all secondary outcomes. As the investigators launch a national advertising campaign for salisartan, independent groups report that the drug is inferior for its primary outcome compared to losartan and associated with respiratory failure among patients with pulmonary hypertension. How could this study have been improved?
- A. Increased study duration
- B. Posthoc analysis of primary outcome among patients who withdrew from study
- C. Randomization (Correct Answer)
- D. Increased sample size
- E. Retrial of primary outcome for clinical effectiveness instead of non-inferiority
Randomized controlled trials Explanation: ***Randomization***
- The study allocated patients **sequentially** (first 1,000 to losartan, next 1,000 to salisartan), introducing **selection bias** as the two groups may not be comparable at baseline for unmeasured confounders.
- **Randomization** ensures that both known and unknown confounding factors are evenly distributed between treatment groups, making the groups comparable and increasing the reliability of the observed treatment effects.
- The lack of randomization explains why independent groups found **different results**—the study's internal validity was compromised by systematic differences between groups that were not due to the intervention itself.
- Sequential allocation is particularly problematic because patient characteristics may **change over time** (e.g., seasonal variations, changes in referral patterns, or evolution in diagnostic criteria).
*Increased study duration*
- While a longer study duration might reveal long-term effects or adverse events, the primary issue of **baseline incomparability** due to the lack of randomization would persist.
- Increasing duration would not address the fundamental flaw in the **patient allocation method** that led to potential bias.
*Posthoc analysis of primary outcome among patients who withdrew from study*
- A **post-hoc analysis** of withdrawn patients would be useful for understanding reasons for withdrawal but cannot correct for the initial lack of randomization or the **attrition bias** caused by the large number of withdrawals (500/2,000 = 25%).
- This approach would also be susceptible to **selection bias** because the reasons for withdrawal might differ between the two groups.
- While **intention-to-treat analysis** was performed, the fundamental allocation bias remains.
*Increased sample size*
- A larger sample size generally increases statistical power and precision, but it does not correct for **systematic errors** introduced by a flawed study design, such as lack of randomization.
- Increasing the sample size would simply replicate the biased allocation across more participants, potentially **amplifying** the effects of selection bias rather than reducing them.
*Retrial of primary outcome for clinical effectiveness instead of non-inferiority*
- Changing the trial design from **non-inferiority** to **superiority** would alter the hypothesis being tested but would not address the underlying methodological flaws.
- The mode of patient allocation (sequential assignment) remains the critical weakness, invalidating any conclusions regarding either non-inferiority or superiority.
- The discrepancy between this study's findings and independent reports highlights that the **study design** (not the research question) was flawed.
Randomized controlled trials US Medical PG Question 3: A research team is studying the effects of a novel drug that was discovered to treat type 2 diabetes. In order to learn more about its effects, they follow patients who are currently taking the drug and determine whether there are adverse effects that exceed anticipated levels and may therefore be drug-related. They discover that the drug causes an excess of sudden cardiac death in 19 patients with renal failure out of 2 million total patients that are followed. Based on these results, an additional warning about this serious adverse effect is added to the investigator brochure for the drug. Which of the following clinical phase studies does this study most likely describe?
- A. Phase IV (Correct Answer)
- B. Phase II
- C. Phase V
- D. Phase III
- E. Phase I
Randomized controlled trials Explanation: ***Phase IV***
- This study occurs **after a drug has been approved and marketed**, focusing on post-marketing surveillance for long-term safety, effectiveness, and real-world side effects in a large and diverse patient population.
- The discovery of a rare but serious adverse effect (sudden cardiac death) in a large patient population (2 million) after the drug is already in use is characteristic of a **Phase IV clinical trial**.
*Phase II*
- Phase II trials involve a **larger group of patients (hundreds)** and focus on evaluating the drug's effectiveness and further assessing safety in patients with the target condition.
- This phase is typically conducted **before widespread marketing** and would not involve 2 million patients.
*Phase V*
- There is **no widely recognized "Phase V"** in standard clinical trial terminology (Phases I-IV focus on drug development and post-marketing surveillance).
- This term is sometimes used informally to refer to **health economics and outcomes research** or implementation studies, which are not described in the scenario.
*Phase III*
- Phase III trials are large-scale studies involving **thousands of patients** to confirm effectiveness, monitor side effects, compare the drug to standard treatments, and collect information for safe use.
- While large, these trials are conducted **before regulatory approval** and marketing, and would not typically follow 2 million patients already taking the drug in the real world.
*Phase I*
- Phase I trials are the **first stage of human testing**, involving a small group of healthy volunteers (20-100) to assess safety, dosage, and pharmacokinetics.
- The primary goal is to determine if the drug is safe enough for further testing, not to identify rare adverse events in a large patient population.
Randomized controlled trials US Medical PG Question 4: You are reading through a recent article that reports significant decreases in all-cause mortality for patients with malignant melanoma following treatment with a novel biological infusion. Which of the following choices refers to the probability that a study will find a statistically significant difference when one truly does exist?
- A. Type II error
- B. Type I error
- C. Confidence interval
- D. p-value
- E. Power (Correct Answer)
Randomized controlled trials Explanation: ***Power***
- **Power** is the probability that a study will correctly reject the null hypothesis when it is, in fact, false (i.e., will find a statistically significant difference when one truly exists).
- A study with high power minimizes the risk of a **Type II error** (failing to detect a real effect).
*Type II error*
- A **Type II error** (or **beta error**) occurs when a study fails to reject a false null hypothesis, meaning it concludes there is no significant difference when one actually exists.
- This is the **opposite** of what the question describes, which asks for the probability of *finding* a difference.
*Type I error*
- A **Type I error** (or **alpha error**) occurs when a study incorrectly rejects a true null hypothesis, concluding there is a significant difference when one does not actually exist.
- This relates to the **p-value** and the level of statistical significance (e.g., p < 0.05).
*Confidence interval*
- A **confidence interval** provides a range of values within which the true population parameter is likely to lie with a certain degree of confidence (e.g., 95%).
- It does not directly represent the probability of finding a statistically significant difference when one truly exists.
*p-value*
- The **p-value** is the probability of observing data as extreme as, or more extreme than, that obtained in the study, assuming the null hypothesis is true.
- It is used to determine statistical significance, but it is not the probability of detecting a true effect.
Randomized controlled trials US Medical PG Question 5: A physician attempts to study cirrhosis in his state. Using a registry of admitted patients over the last 10 years at the local hospital, he isolates all patients who have been diagnosed with cirrhosis. Subsequently, he contacts this group of patients, asking them to complete a survey assessing their prior exposure to alcohol use, intravenous drug abuse, blood transfusions, personal history of cancer, and other medical comorbidities. An identical survey is given to an equal number of patients in the registry who do not carry a prior diagnosis of cirrhosis. Which of the following is the study design utilized by this physician?
- A. Randomized controlled trial
- B. Case-control study (Correct Answer)
- C. Cross-sectional study
- D. Cohort study
- E. Meta-analysis
Randomized controlled trials Explanation: ***Case-control study***
- This study design **identifies subjects based on their outcome (cases with cirrhosis, controls without cirrhosis)** and then retrospectively investigates their past exposures.
- The physician selected patients with cirrhosis (cases) and patients without cirrhosis (controls), then assessed their prior exposures to risk factors like alcohol use and intravenous drug abuse.
*Randomized controlled trial*
- This design involves randomly assigning participants to an **intervention group** or a **control group** to assess the effect of an intervention.
- There is no intervention being tested or randomization occurring in this study; it is observational.
*Cross-sectional study*
- A cross-sectional study measures the **prevalence of disease and exposure at a single point in time** in a defined population.
- This study collects retrospective exposure data and compares two distinct groups (cases and controls), rather than assessing prevalence at one time point.
*Cohort study*
- A cohort study **follows a group of individuals over time** to see if their exposure to a risk factor is associated with the development of a disease.
- This study starts with the outcome (cirrhosis) and looks backward at exposures, which is the opposite direction of a cohort study.
*Meta-analysis*
- A meta-analysis is a statistical method that **combines the results of multiple independent studies** to produce a single, more powerful estimate of treatment effect or association.
- This is an original research study collecting new data, not a systematic review or synthesis of existing studies.
Randomized controlled trials US Medical PG Question 6: You are currently employed as a clinical researcher working on clinical trials of a new drug to be used for the treatment of Parkinson's disease. Currently, you have already determined the safe clinical dose of the drug in a healthy patient. You are in the phase of drug development where the drug is studied in patients with the target disease to determine its efficacy. Which of the following phases is this new drug currently in?
- A. Phase 4
- B. Phase 1
- C. Phase 2 (Correct Answer)
- D. Phase 0
- E. Phase 3
Randomized controlled trials Explanation: ***Phase 2***
- **Phase 2 trials** involve studying the drug in patients with the target disease to assess its **efficacy** and further evaluate safety, typically involving a few hundred patients.
- The question describes a stage after safe dosing in healthy patients (Phase 1) and before large-scale efficacy confirmation (Phase 3), focusing on efficacy in the target population.
*Phase 4*
- **Phase 4 trials** occur **after a drug has been approved** and marketed, monitoring long-term effects, optimal use, and rare side effects in a diverse patient population.
- This phase is conducted post-market approval, whereas the question describes a drug still in development prior to approval.
*Phase 1*
- **Phase 1 trials** primarily focus on determining the **safety and dosage** of a new drug in a **small group of healthy volunteers** (or sometimes patients with advanced disease if the drug is highly toxic).
- The question states that the safe clinical dose in a healthy patient has already been determined, indicating that Phase 1 has been completed.
*Phase 0*
- **Phase 0 trials** are exploratory, very early-stage studies designed to confirm that the drug reaches the target and acts as intended, typically involving a very small number of doses and participants.
- These trials are conducted much earlier in the development process, preceding the determination of safe clinical doses and large-scale efficacy studies.
*Phase 3*
- **Phase 3 trials** are large-scale studies involving hundreds to thousands of patients to confirm **efficacy**, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
- While Phase 3 does assess efficacy, it follows Phase 2 and is typically conducted on a much larger scale before submitting for regulatory approval.
Randomized controlled trials US Medical PG Question 7: In a randomized controlled trial studying a new treatment, the primary endpoint (mortality) occurred in 14.4% of the treatment group and 16.7% of the control group. Which of the following represents the number of patients needed to treat to save one life, based on the primary endpoint?
- A. 1/(0.144 - 0.167)
- B. 1/(0.167 - 0.144) (Correct Answer)
- C. 1/(0.300 - 0.267)
- D. 1/(0.267 - 0.300)
- E. 1/(0.136 - 0.118)
Randomized controlled trials Explanation: ***1/(0.167 - 0.144)***
- The **Number Needed to Treat (NNT)** is calculated as **1 / Absolute Risk Reduction (ARR)**.
- The **Absolute Risk Reduction (ARR)** is the difference between the event rate in the control group (16.7%) and the event rate in the treatment group (14.4%), which is **0.167 - 0.144**.
*1/(0.144 - 0.167)*
- This calculation represents 1 divided by the **Absolute Risk Increase**, which would be relevant if the treatment increased mortality.
- The **NNT should always be a positive value**, indicating the number of patients to treat to prevent one adverse event.
*1/(0.300 - 0.267)*
- This option uses arbitrary numbers (0.300 and 0.267) that do not correspond to the given **mortality rates** in the problem.
- It does not reflect the correct calculation for **absolute risk reduction** based on the provided data.
*1/(0.267 - 0.300)*
- This option also uses arbitrary numbers not derived from the problem's data, and it would result in a **negative value** for the denominator.
- The difference between event rates of 0.267 and 0.300 is not present in the given information for this study.
*1/(0.136 - 0.118)*
- This calculation uses arbitrary numbers (0.136 and 0.118) that are not consistent with the reported **mortality rates** of 14.4% and 16.7%.
- These values do not represent the **Absolute Risk Reduction** required for calculating NNT in this specific scenario.
Randomized controlled trials US Medical PG Question 8: A scientist is designing a study to determine whether eating a new diet is able to lower blood pressure in a group of patients. In particular, he believes that starting the diet may help decrease peak blood pressures throughout the day. Therefore, he will equip study participants with blood pressure monitors and follow pressure trends over a 24-hour period. He decides that after recruiting subjects, he will start them on either the new diet or a control diet and follow them for 1 month. After this time, he will switch patients onto the other diet and follow them for an additional month. He will analyze the results from the first month against the results from the second month for each patient. This type of study design is best at controlling for which of the following problems with studies?
- A. Hawthorne effect
- B. Recall bias
- C. Confounding (Correct Answer)
- D. Selection bias
- E. Pygmalion effect
Randomized controlled trials Explanation: ***Confounding***
- This **crossover design** (switching patients to the other diet) effectively controls for **confounding variables** by making each patient their own control, ensuring that inherent patient characteristics do not bias the comparison between diets.
- By comparing the effects of both diets within the same individual, individual variability in factors such as genetics, lifestyle, and other co-morbidities are accounted for, reducing their potential as confounders.
*Hawthorne effect*
- The **Hawthorne effect** refers to subjects modifying their behavior in response to being observed, which this study design does not specifically address or eliminate.
- While patients are being monitored, the design aims to compare the diets' effects, not to prevent behavioral changes due to observation itself.
*Recall bias*
- **Recall bias** occurs when participants' memories of past events are inaccurate, often influenced by their current health status or beliefs.
- This study measures **real-time blood pressure** data, not relying on recollection of past exposures or outcomes, thereby mitigating recall bias.
*Selection bias*
- **Selection bias** arises from non-random selection of participants into study groups, leading to systematic differences between groups.
- While patient recruitment could introduce selection bias into the overall study population, the **crossover design** itself helps control for differences between treatment arms because all participants eventually receive both treatments.
*Pygmalion effect*
- The **Pygmalion effect** (or observer-expectancy effect) describes phenomena where higher expectations lead to increased performance, usually from a researcher influencing a subject.
- This effect is not directly addressed by the crossover design; the design focuses on controlling for patient-specific confounders rather than investigator bias in expectations.
Randomized controlled trials US Medical PG Question 9: Researchers are studying the relationship between heart disease and alcohol consumption. They review the electronic medical records of 500 patients at a local hospital during the study period and identify the presence or absence of acute coronary syndrome (ACS) and the number of alcoholic drinks consumed on the day of presentation. They find that there is a lower prevalence of acute coronary syndrome in patients who reported no alcohol consumption or 1 drink daily compared with those who reported 2 or more drinks. Which of the following is the most accurate description of this study type?
- A. Cross-sectional study
- B. Prospective study
- C. Randomized controlled trial
- D. Case-control study
- E. Retrospective study (Correct Answer)
Randomized controlled trials Explanation: ***Retrospective study***
- This study **reviews electronic medical records** that were created in the past, making it retrospective by definition.
- Researchers looked **backward in time** during the study period to identify both the exposure (alcohol consumption) and outcome (ACS) from existing records.
- The key feature is that **data collection relies on pre-existing documentation** rather than prospectively following patients or collecting data at a single point in time.
- This is specifically a **retrospective cohort design** where researchers identified a population and assessed both exposure and outcome from historical records.
*Cross-sectional study*
- Cross-sectional studies collect data from participants at a **single point in time** through surveys, interviews, or direct assessment—not by reviewing past medical records.
- While this study assessed variables "at presentation," the **method of data collection** (reviewing electronic records retrospectively) makes it retrospective, not cross-sectional.
- Cross-sectional studies typically involve **active data collection** from living participants, not record review.
*Prospective study*
- A prospective study follows participants **forward in time** from exposure to outcome, recruiting them before outcomes develop.
- This study did not follow patients forward; it reviewed **records of events that already occurred**.
*Randomized controlled trial*
- An RCT involves **intervention and randomization** of participants to different treatment groups.
- This is an observational study with no intervention or randomization.
*Case-control study*
- A case-control study first identifies **cases (with disease)** and **controls (without disease)**, then looks backward to compare exposures.
- This study did not select participants based on disease status first; it reviewed a general hospital population and assessed both variables simultaneously from records.
Randomized controlled trials US Medical PG Question 10: A 16-year-old girl comes to the physician because she is worried about gaining weight. She reports that at least twice a week, she eats excessive amounts of food but feels ashamed about losing control soon after. She is very active in her high school's tennis team and goes running daily to lose weight. She has a history of cutting her forearms with the metal tab from a soda can. Her last menstrual period was 3 weeks ago. She is 165 cm (5 ft 5 in) tall and weighs 57 kg (125 lb); BMI is 21 kg/m2. Physical examination shows enlarged, firm parotid glands bilaterally. There are erosions of the enamel on the lingual surfaces of the teeth. Which of the following is the most likely diagnosis?
- A. Borderline personality disorder
- B. Bulimia nervosa (Correct Answer)
- C. Body dysmorphic disorder
- D. Anorexia nervosa
- E. Obsessive-compulsive disorder
Randomized controlled trials Explanation: ***Bulimia nervosa***
- This patient exhibits characteristic features of bulimia nervosa, including recurrent episodes of **binge eating** (at least twice weekly) followed by inappropriate **compensatory behaviors**.
- The **bilateral parotid gland enlargement** and **lingual enamel erosion** are **pathognomonic physical signs of chronic self-induced vomiting** (purging behavior), combined with excessive exercise as additional compensation.
- Her normal BMI of 21 kg/m² is highly consistent with bulimia nervosa, as individuals with this condition typically maintain a **normal weight or are overweight**, unlike those with anorexia nervosa.
- The sense of **loss of control** and **shame** about eating episodes are core features of this disorder.
*Borderline personality disorder*
- While **self-harm** (cutting) can be associated with borderline personality disorder, the primary concern in this patient is the prominent eating disorder symptoms with pathognomonic physical findings.
- Borderline personality disorder is characterized by a pervasive pattern of **instability in interpersonal relationships**, self-image, affects, and marked impulsivity; these are not the main presenting complaints here.
- Self-harm behavior can occur in multiple psychiatric conditions and does not alone establish this diagnosis.
*Body dysmorphic disorder*
- This disorder involves a **preoccupation with perceived flaws in physical appearance** that are minimal or unobservable to others, leading to significant distress or impairment.
- While the patient is concerned about gaining weight, her primary symptoms revolve around **binge-purge cycles** with physical evidence of purging behavior, rather than a sole preoccupation with a specific body defect.
*Anorexia nervosa*
- Anorexia nervosa is characterized by **restriction of energy intake** leading to a significantly low body weight (BMI usually <17.5 kg/m²) and intense fear of gaining weight despite being underweight.
- This patient has a **normal BMI (21 kg/m²)** and engages in binge-eating followed by compensatory behaviors (purging and exercise), which represents bulimia nervosa rather than anorexia nervosa.
- Additionally, she has **regular menses** (last period 3 weeks ago), whereas amenorrhea is common in anorexia nervosa due to low body weight.
*Obsessive-compulsive disorder*
- OCD involves recurrent, persistent, **intrusive thoughts (obsessions)** and/or repetitive behaviors or mental acts that an individual feels driven to perform **(compulsions)** to reduce anxiety.
- While some of the patient's behaviors might seem ritualistic, the core symptoms are clearly related to **eating disorder pathology with binge-purge cycles**, not typical OCD themes like contamination, symmetry, or checking behaviors.
- The physical signs of chronic purging behavior definitively point to an eating disorder diagnosis.
More Randomized controlled trials US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.