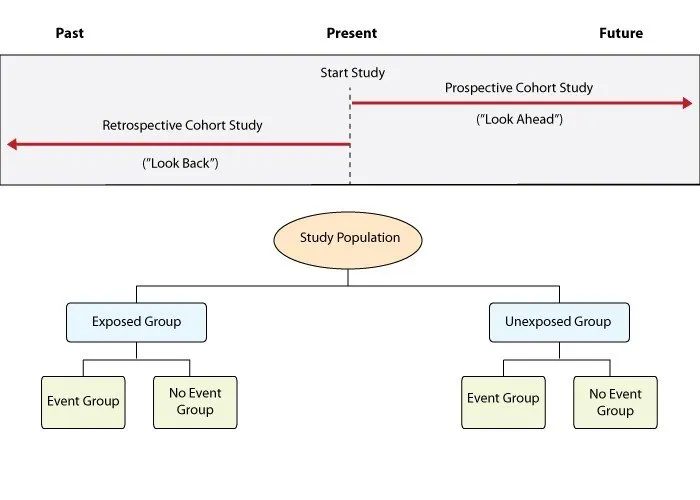
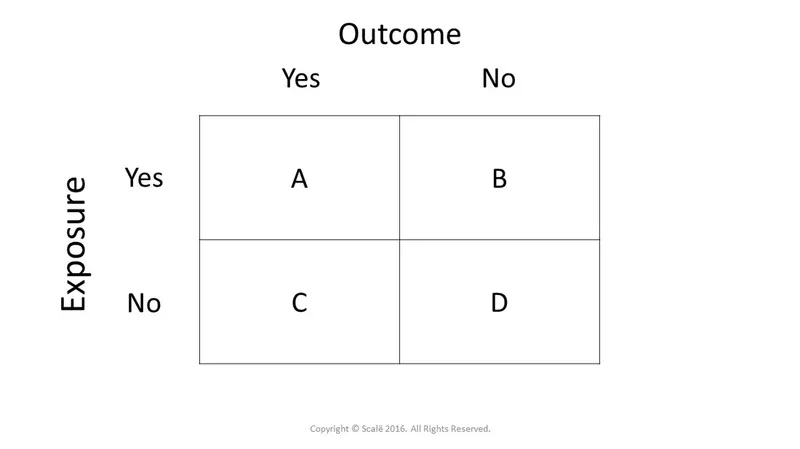
Cohort studies US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Cohort studies. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Cohort studies US Medical PG Question 1: A researcher is studying whether a new knee implant is better than existing alternatives in terms of pain after knee replacement. She designs the study so that it includes all the surgeries performed at a certain hospital. Interestingly, she notices that patients who underwent surgeries on Mondays and Thursdays reported much better pain outcomes on a survey compared with those who underwent the same surgeries from the same surgeons on Tuesdays and Fridays. Upon performing further analysis, she discovers that one of the staff members who works on Mondays and Thursdays is aware of the study and tells all the patients about how wonderful the new implant is. Which of the following forms of bias does this most likely represent?
- A. Hawthorne effect
- B. Pygmalion effect (Correct Answer)
- C. Attrition bias
- D. Golem effect
Cohort studies Explanation: ***Pygmalion effect***
- This bias occurs when higher expectations lead to an increase in performance. In this scenario, the staff member's positive reinforcement about the new implant likely instilled **higher patient expectations**, leading to better reported pain outcomes.
- The patients' belief in the implant's superiority, influenced by the staff member, acted as a **self-fulfilling prophecy**, improving their subjective pain experience.
*Hawthorne effect*
- This effect describes how individuals modify an aspect of their behavior in response to their awareness of being observed. While patients were part of a study, their improved outcomes were specifically linked to a staff member's verbal influence, not solely the act of observation.
- The improved pain outcomes stem from the **expectations created by the staff member's praise**, rather than a general awareness of being studied.
*Attrition bias*
- Attrition bias refers to systematic differences between groups in the loss of participants from a study.
- This scenario describes differences in patient outcomes based on staff influence during the study, not due to **patients dropping out differentially** between groups.
*Golem effect*
- The Golem effect is the opposite of the Pygmalion effect, where lower expectations placed upon individuals lead to poorer performance from them.
- In this case, the staff member's influence created **high expectations and positive outcomes**, not negative expectations leading to worse outcomes.
Cohort studies US Medical PG Question 2: You have been entrusted with the task of finding the causes of low birth weight in infants born in the health jurisdiction for which you are responsible. In 2017, there were 1,500 live births and, upon further inspection of the birth certificates, 108 of these children had a low birth weight (i.e. lower than 2,500 g), while 237 had mothers who smoked continuously during pregnancy. Further calculations have shown that the risk of low birth weight in smokers was 14% and in non-smokers, it was 7%, while the relative risk of low birth weight linked to cigarette smoking during pregnancy was 2%. In other words, women who smoked during pregnancy were twice as likely as those who did not smoke to deliver a low-weight infant. Using this data, you are also asked to calculate how much of the excess risk for low birth weight, in percentage terms, can be attributed to smoking. What is the attributable risk percentage for smoking leading to low birth weight?
- A. 40%
- B. 30%
- C. 20%
- D. 10%
- E. 50% (Correct Answer)
Cohort studies Explanation: ***50%***
- This value is calculated using the formula for **attributable risk percent (ARP)** in the exposed group: ARP = ((Risk in exposed - Risk in unexposed) / Risk in exposed) × 100.
- Given that the risk of low birth weight in smokers (exposed) is 14% and in non-smokers (unexposed) is 7%, the calculation is ((0.14 - 0.07) / 0.14) × 100 = (0.07 / 0.14) × 100 = **0.50 × 100 = 50%**.
*40%*
- This percentage does not align with the provided risk values for low birth weight in smokers (14%) and non-smokers (7%).
- A calculation of ((0.14 - 0.07) / 0.14) * 100 does not yield 40%.
*30%*
- This value is incorrect, as it would suggest a smaller difference in risk between the exposed and unexposed groups relative to the risk in the exposed group than what is presented in the problem.
- The calculated attributable risk percent is higher than 30%.
*20%*
- This option is significantly lower than the true attributable risk percent derived from the given risk figures.
- It would imply a much weaker association between smoking and low birth weight in terms of excess risk than what is calculated.
*10%*
- This value is substantially different from the correct calculation and would suggest a very minor attributable risk.
- The attributable risk percent for smoking leading to low birth weight is much higher than 10% based on the provided data.
Cohort studies US Medical PG Question 3: A research team develops a new monoclonal antibody checkpoint inhibitor for advanced melanoma that has shown promise in animal studies as well as high efficacy and low toxicity in early phase human clinical trials. The research team would now like to compare this drug to existing standard of care immunotherapy for advanced melanoma. The research team decides to conduct a non-randomized study where the novel drug will be offered to patients who are deemed to be at risk for toxicity with the current standard of care immunotherapy, while patients without such risk factors will receive the standard treatment. Which of the following best describes the level of evidence that this study can offer?
- A. Level 1
- B. Level 3 (Correct Answer)
- C. Level 5
- D. Level 4
- E. Level 2
Cohort studies Explanation: ***Level 3***
- A **non-randomized controlled trial** like the one described, where patient assignment to treatment groups is based on specific characteristics (risk of toxicity), falls into Level 3 evidence.
- This level typically includes **non-randomized controlled trials** and **well-designed cohort studies** with comparison groups, which are prone to selection bias and confounding.
- The study compares two treatments but lacks randomization, making it Level 3 evidence.
*Level 1*
- Level 1 evidence is the **highest level of evidence**, derived from **systematic reviews and meta-analyses** of multiple well-designed randomized controlled trials or large, high-quality randomized controlled trials.
- The described study is explicitly stated as non-randomized, ruling out Level 1.
*Level 2*
- Level 2 evidence involves at least one **well-designed randomized controlled trial** (RCT) or **systematic reviews** of randomized trials.
- The current study is *non-randomized*, which means it cannot be classified as Level 2 evidence, as randomization is a key criterion for this level.
*Level 4*
- Level 4 evidence includes **case series**, **case-control studies**, and **poorly designed cohort or case-control studies**.
- While the study is non-randomized, it is a controlled comparative trial rather than a case series or retrospective case-control study, placing it at Level 3.
*Level 5*
- Level 5 evidence is the **lowest level of evidence**, typically consisting of **expert opinion** without explicit critical appraisal, or based on physiology, bench research, or animal studies.
- While the drug was initially tested in animal studies, the current human comparative study offers a higher level of evidence than expert opinion or preclinical data.
Cohort studies US Medical PG Question 4: A 25-year-old man with a genetic disorder presents for genetic counseling because he is concerned about the risk that any children he has will have the same disease as himself. Specifically, since childhood he has had difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy. He has also had diarrhea and malabsorption requiring enzyme replacement therapy. If his wife comes from a population where 1 in 10,000 people are affected by this same disorder, which of the following best represents the likelihood a child would be affected as well?
- A. 0.01%
- B. 2%
- C. 0.5%
- D. 1% (Correct Answer)
- E. 50%
Cohort studies Explanation: ***Correct Option: 1%***
- The patient's symptoms (difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy; diarrhea and malabsorption requiring enzyme replacement therapy) are classic for **cystic fibrosis (CF)**, an **autosomal recessive disorder**.
- For an autosomal recessive disorder with a prevalence of 1 in 10,000 in the general population, **q² = 1/10,000**, so **q = 1/100 = 0.01**. The carrier frequency **(2pq)** is approximately **2q = 2 × (1/100) = 1/50 = 0.02**.
- The affected man is **homozygous recessive (aa)** and will always pass on the recessive allele. His wife has a **1/50 chance of being a carrier (Aa)**. If she is a carrier, she has a **1/2 chance of passing on the recessive allele**.
- Therefore, the probability of an affected child = **(Probability wife is a carrier) × (Probability wife passes recessive allele) = 1/50 × 1/2 = 1/100 = 1%**.
*Incorrect Option: 0.01%*
- This percentage is too low and does not correctly account for the carrier frequency in the population and the probability of transmission from a carrier mother.
*Incorrect Option: 2%*
- This represents approximately the carrier frequency (1/50 ≈ 2%), but does not account for the additional 1/2 probability that a carrier mother would pass on the recessive allele.
*Incorrect Option: 0.5%*
- This value would be correct if the carrier frequency were 1/100 instead of 1/50, which does not match the given population prevalence.
*Incorrect Option: 50%*
- **50%** would be the risk if both parents were carriers of an autosomal recessive disorder (1/4 chance = 25% for affected, but if we know one parent passes the allele, conditional probability changes). More accurately, 50% would apply if the disorder were **autosomal dominant** with one affected parent, which is not the case here.
Cohort studies US Medical PG Question 5: A recent study attempted to analyze whether increased "patient satisfaction" driven healthcare resulted in increased hospitalization. Using this patient population, the sociodemographics, health status, and hospital use were assessed. Next year, patient satisfaction with health care providers was assessed using 5 items from the Consumer Assessment of Health Plans Survey. Which of the following best describes this study design?
- A. Retrospective case-control
- B. Cross-sectional study
- C. Prospective case-control
- D. Retrospective cohort
- E. Prospective cohort (Correct Answer)
Cohort studies Explanation: ***Prospective cohort***
- This study collects baseline data (sociodemographics, health status, hospital use) on a patient population and then follows them forward in time to assess patient satisfaction the following year. This forward-looking approach with follow-up over time defines a **prospective cohort study**.
- The study establishes a cohort at baseline, measures initial characteristics and hospital use, then prospectively assesses patient satisfaction and subsequent healthcare utilization, allowing analysis of associations between satisfaction and hospitalization patterns.
*Retrospective case-control*
- A **case-control study** identifies individuals with an outcome (cases) and without the outcome (controls) and then looks backward in time to determine past exposures.
- This study does not select participants based on outcome status; instead, it defines a cohort and follows them forward, which is characteristic of cohort design, not case-control.
*Cross-sectional study*
- A **cross-sectional study** measures both exposure and outcome at a single point in time, providing a snapshot of the population.
- This study involves follow-up over time, as patient satisfaction is assessed "next year" after baseline data collection, making it longitudinal rather than cross-sectional.
*Prospective case-control*
- **Case-control studies** inherently select participants based on their outcome status (cases vs. controls), whether prospective or retrospective.
- This study starts with a defined patient population before outcomes occur and follows them forward without outcome-based selection, which is characteristic of a cohort study, not a case-control design.
*Retrospective cohort*
- A **retrospective cohort study** uses existing data to define a cohort and then looks back in time to identify exposures and outcomes that have already occurred.
- This study involves collecting new data prospectively and following participants forward ("next year"), rather than analyzing past records, making it prospective rather than retrospective.
Cohort studies US Medical PG Question 6: A researcher is designing an experiment to examine the toxicity of a new chemotherapeutic agent in mice. She splits the mice into 2 groups, one of which she exposes to daily injections of the drug for 1 week. The other group is not exposed to any intervention. Both groups are otherwise raised in the same conditions with the same diet. One month later, she sacrifices the mice to check for dilated cardiomyopathy. In total, 52 mice were exposed to the drug, and 50 were not exposed. Out of the exposed group, 13 were found to have dilated cardiomyopathy on necropsy. In the unexposed group, 1 mouse was found to have dilated cardiomyopathy. Which of the following is the relative risk of developing cardiomyopathy with this drug?
- A. 12.5 (Correct Answer)
- B. 25.0
- C. 13.7
- D. 16.3
- E. 23.0
Cohort studies Explanation: ***Correct Option: 12.5***
- The **relative risk (RR)** is calculated as the **risk in the exposed group divided by the risk in the unexposed group**: RR = [a/(a+b)] / [c/(c+d)]
- **Risk in exposed group** = 13/52 = 0.25 (25%)
- **Risk in unexposed group** = 1/50 = 0.02 (2%)
- **RR = 0.25 / 0.02 = 12.5**
- This indicates that mice exposed to the chemotherapeutic agent are **12.5 times more likely** to develop dilated cardiomyopathy compared to unexposed mice
- An **RR > 1** indicates increased risk with exposure, supporting the drug's cardiotoxicity
*Incorrect Option: 25.0*
- This value results from **miscalculating the unexposed group risk** (e.g., using 0.01 instead of 0.02 as the denominator)
- If the unexposed risk was halved incorrectly: 0.25 / 0.01 = 25.0
- This overestimates the relative risk by a factor of 2
*Incorrect Option: 13.7*
- This value does not result from the correct **relative risk formula**
- May arise from an **arithmetic error** or confusion with other epidemiological measures
- The correct calculation of 13/52 ÷ 1/50 does not yield this result
*Incorrect Option: 16.3*
- This might result from **miscounting the number of subjects** in either group or confusing **relative risk with odds ratio**
- The **odds ratio** would be calculated as (13/39) / (1/49) = 16.3
- Remember: **Relative risk uses total exposed/unexposed as denominators**, while odds ratio uses non-diseased counts
*Incorrect Option: 23.0*
- This value suggests a **fundamental error** in applying the relative risk formula
- Could result from using incorrect numerators or denominators (e.g., 13/1 instead of proper risk calculation)
- Significantly overestimates the true relative risk of 12.5
Cohort studies US Medical PG Question 7: You are reading through a recent article that reports significant decreases in all-cause mortality for patients with malignant melanoma following treatment with a novel biological infusion. Which of the following choices refers to the probability that a study will find a statistically significant difference when one truly does exist?
- A. Type II error
- B. Type I error
- C. Confidence interval
- D. p-value
- E. Power (Correct Answer)
Cohort studies Explanation: ***Power***
- **Power** is the probability that a study will correctly reject the null hypothesis when it is, in fact, false (i.e., will find a statistically significant difference when one truly exists).
- A study with high power minimizes the risk of a **Type II error** (failing to detect a real effect).
*Type II error*
- A **Type II error** (or **beta error**) occurs when a study fails to reject a false null hypothesis, meaning it concludes there is no significant difference when one actually exists.
- This is the **opposite** of what the question describes, which asks for the probability of *finding* a difference.
*Type I error*
- A **Type I error** (or **alpha error**) occurs when a study incorrectly rejects a true null hypothesis, concluding there is a significant difference when one does not actually exist.
- This relates to the **p-value** and the level of statistical significance (e.g., p < 0.05).
*Confidence interval*
- A **confidence interval** provides a range of values within which the true population parameter is likely to lie with a certain degree of confidence (e.g., 95%).
- It does not directly represent the probability of finding a statistically significant difference when one truly exists.
*p-value*
- The **p-value** is the probability of observing data as extreme as, or more extreme than, that obtained in the study, assuming the null hypothesis is true.
- It is used to determine statistical significance, but it is not the probability of detecting a true effect.
Cohort studies US Medical PG Question 8: A research group wants to assess the safety and toxicity profile of a new drug. A clinical trial is conducted with 20 volunteers to estimate the maximum tolerated dose and monitor the apparent toxicity of the drug. The study design is best described as which of the following phases of a clinical trial?
- A. Phase 0
- B. Phase III
- C. Phase V
- D. Phase II
- E. Phase I (Correct Answer)
Cohort studies Explanation: ***Phase I***
- **Phase I clinical trials** involve a small group of healthy volunteers (typically 20-100) to primarily assess **drug safety**, determine a safe dosage range, and identify side effects.
- The main goal is to establish the **maximum tolerated dose (MTD)** and evaluate the drug's pharmacokinetic and pharmacodynamic profiles.
*Phase 0*
- **Phase 0 trials** are exploratory studies conducted in a very small number of subjects (10-15) to gather preliminary data on a drug's **pharmacodynamics and pharmacokinetics** in humans.
- They involve microdoses, not intended to have therapeutic effects, and thus cannot determine toxicity or MTD.
*Phase III*
- **Phase III trials** are large-scale studies involving hundreds to thousands of patients to confirm the drug's **efficacy**, monitor side effects, compare it to standard treatments, and collect information that will allow the drug to be used safely.
- These trials are conducted after safety and initial efficacy have been established in earlier phases.
*Phase V*
- "Phase V" is not a standard, recognized phase in the traditional clinical trial classification (Phase 0, I, II, III, IV).
- This term might be used in some non-standard research contexts or for post-marketing studies that go beyond Phase IV surveillance, but it is not a formal phase for initial drug development.
*Phase II*
- **Phase II trials** involve several hundred patients with the condition the drug is intended to treat, focusing on **drug efficacy** and further evaluating safety.
- While safety is still monitored, the primary objective shifts to determining if the drug works for its intended purpose and at what dose.
Cohort studies US Medical PG Question 9: A 28-year-old male presents to his primary care physician with complaints of intermittent abdominal pain and alternating bouts of constipation and diarrhea. His medical chart is not significant for any past medical problems or prior surgeries. He is not prescribed any current medications. Which of the following questions would be the most useful next question in eliciting further history from this patient?
- A. "Does the diarrhea typically precede the constipation, or vice-versa?"
- B. "Is the diarrhea foul-smelling?"
- C. "Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life"
- D. "Are the symptoms worse in the morning or at night?"
- E. "Can you tell me more about the symptoms you have been experiencing?" (Correct Answer)
Cohort studies Explanation: ***Can you tell me more about the symptoms you have been experiencing?***
- This **open-ended question** encourages the patient to provide a **comprehensive narrative** of their symptoms, including details about onset, frequency, duration, alleviating/aggravating factors, and associated symptoms, which is crucial for diagnosis.
- In a patient presenting with vague, intermittent symptoms like alternating constipation and diarrhea, allowing them to elaborate freely can reveal important clues that might not be captured by more targeted questions.
*Does the diarrhea typically precede the constipation, or vice-versa?*
- While knowing the sequence of symptoms can be helpful in understanding the **pattern of bowel dysfunction**, it is a very specific question that might overlook other important aspects of the patient's experience.
- It prematurely narrows the focus without first obtaining a broad understanding of the patient's overall symptomatic picture.
*Is the diarrhea foul-smelling?*
- Foul-smelling diarrhea can indicate **malabsorption** or **bacterial overgrowth**, which are important to consider in some gastrointestinal conditions.
- However, this is a **specific symptom inquiry** that should follow a more general exploration of the patient's symptoms, as it may not be relevant if other crucial details are missed.
*Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life*
- Quantifying pain intensity is useful for assessing the **severity of discomfort** and monitoring changes over time.
- However, for a patient with intermittent rather than acute, severe pain, understanding the **character, location, and triggers** of the pain is often more diagnostically valuable than just a numerical rating initially.
*Are the symptoms worse in the morning or at night?*
- Diurnal variation can be relevant in certain conditions, such as inflammatory bowel diseases where nocturnal symptoms might be more concerning, or functional disorders whose symptoms might be stress-related.
- This is another **specific question** that should come after gathering a more complete initial picture of the patient's symptoms to ensure no key information is overlooked.
Cohort studies US Medical PG Question 10: You are interested in studying the etiology of heart failure reduced ejection fraction (HFrEF) and attempt to construct an appropriate design study. Specifically, you wish to look for potential causality between dietary glucose consumption and HFrEF. Which of the following study designs would allow you to assess for and determine this causality?
- A. Cross-sectional study
- B. Case series
- C. Cohort study (Correct Answer)
- D. Case-control study
- E. Randomized controlled trial
Cohort studies Explanation: ***Cohort study***
- A **cohort study** observes a group of individuals over time to identify risk factors and outcomes, allowing for the assessment of **temporal relationships** between exposure (dietary glucose) and outcome (HFrEF).
- This design is suitable for establishing a potential **causal link** as it tracks participants from exposure to outcome, enabling the calculation of incidence rates and relative risks.
*Cross-sectional study*
- A **cross-sectional study** measures exposure and outcome simultaneously at a single point in time, making it impossible to determine the **temporal sequence** of events.
- This design can only identify **associations** or correlations, not causation, as it cannot establish whether high glucose consumption preceded HFrEF.
*Case series*
- A **case series** describes characteristics of a group of patients with a particular disease or exposure, often to highlight unusual clinical features, but it lacks a **comparison group**.
- It cannot assess causality because it does not provide information on the frequency of exposure in healthy individuals or the incidence of the disease in unexposed individuals.
*Case-control study*
- A **case-control study** compares individuals with the outcome (cases) to those without the outcome (controls) to determine past exposures, which makes it prone to **recall bias**.
- While it can suggest associations, it cannot definitively establish a temporal relationship or causation as the outcome is already known when exposure is assessed.
*Randomized controlled trial*
- A **randomized controlled trial (RCT)** is the gold standard for establishing causation by randomly assigning participants to an intervention or control group, but it may not be ethical or feasible for studying long-term dietary exposures and chronic diseases like HFrEF due to the long follow-up period and complexity of diet.
- While ideal for causality, directly controlling and randomizing dietary glucose intake over decades to observe HFrEF development might be practically challenging or unethical.
More Cohort studies US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.