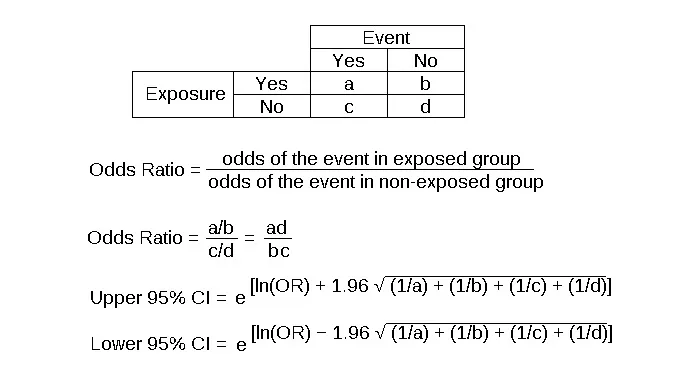
Case-control studies US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Case-control studies. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Case-control studies US Medical PG Question 1: A study is funded by the tobacco industry to examine the association between smoking and lung cancer. They design a study with a prospective cohort of 1,000 smokers between the ages of 20-30. The length of the study is five years. After the study period ends, they conclude that there is no relationship between smoking and lung cancer. Which of the following study features is the most likely reason for the failure of the study to note an association between tobacco use and cancer?
- A. Late-look bias
- B. Latency period (Correct Answer)
- C. Confounding
- D. Effect modification
- E. Pygmalion effect
Case-control studies Explanation: ***Latency period***
- **Lung cancer** typically has a **long latency period**, often **20-30+ years**, between initial exposure to tobacco carcinogens and the development of clinically detectable disease.
- A **five-year study duration** in young smokers (ages 20-30) is **far too short** to observe the development of lung cancer, which explains the false negative finding.
- This represents a **fundamental flaw in study design** rather than a bias—the biological timeline of disease development was not adequately considered.
*Late-look bias*
- **Late-look bias** occurs when a study enrolls participants who have already survived the early high-risk period of a disease, leading to **underestimation of true mortality or incidence**.
- Also called **survival bias**, it involves studying a population that has already been "selected" by survival.
- This is not applicable here, as the study simply ended before sufficient time elapsed for disease to develop.
*Confounding*
- **Confounding** occurs when a third variable is associated with both the exposure and outcome, distorting the apparent relationship between them.
- While confounding can affect study results, it would not completely eliminate the detection of a strong, well-established association like smoking and lung cancer in a properly conducted prospective cohort study.
- The issue here is temporal (insufficient follow-up time), not the presence of an unmeasured confounder.
*Effect modification*
- **Effect modification** (also called interaction) occurs when the magnitude of an association between exposure and outcome differs across levels of a third variable.
- This represents a **true biological phenomenon**, not a study design flaw or bias.
- It would not explain the complete failure to detect any association.
*Pygmalion effect*
- The **Pygmalion effect** (observer-expectancy effect) refers to a psychological phenomenon where higher expectations lead to improved performance in the observed subjects.
- This concept is relevant to **behavioral and educational research**, not to objective epidemiological studies of disease incidence.
- It has no relevance to the biological relationship between carcinogen exposure and cancer development.
Case-control studies US Medical PG Question 2: You have been asked to quantify the relative risk of developing bacterial meningitis following exposure to a patient with active disease. You analyze 200 patients in total, half of which are controls. In the trial arm, 30% of exposed patients ultimately contracted bacterial meningitis. In the unexposed group, only 1% contracted the disease. Which of the following is the relative risk due to disease exposure?
- A. (30 * 99) / (70 * 1)
- B. [30 / (30 + 70)] / [1 / (1 + 99)] (Correct Answer)
- C. [70 / (30 + 70)] / [99 / (1 + 99)]
- D. [[1 / (1 + 99)] / [30 / (30 + 70)]]
- E. (70 * 1) / (30 * 99)
Case-control studies Explanation: ***[30 / (30 + 70)] / [1 / (1 + 99)]***
- This formula correctly calculates the **relative risk (RR)**. The numerator represents the **incidence rate in the exposed group** (30% of 100 exposed patients = 30 cases out of 100), and the denominator represents the **incidence rate in the unexposed group** (1% of 100 unexposed patients = 1 case out of 100).
- Relative risk is the ratio of the **risk of an event** in an **exposed group** to the **risk of an event** in an **unexposed group**.
*[(30 * 99) / (70 * 1)]*
- This formula is for calculating the **odds ratio (OR)**, specifically using a 2x2 table setup where 30 represents exposed cases, 70 represents exposed non-cases, 1 represents unexposed cases, and 99 represents unexposed non-cases.
- The odds ratio is a measure of association between an exposure and an outcome, representing the **odds of an outcome** given exposure compared to the odds of the outcome without exposure.
*[70 / (30 + 70)] / [99 / (1 + 99)]*
- This formula calculates the **relative risk of *not* developing the disease**, which is the inverse of what the question asks for.
- It compares the proportion of exposed individuals who *do not* contract the disease to the proportion of unexposed individuals who *do not* contract the disease.
*[[1 / (1 + 99)] / [30 / (30 + 70)]]*
- This formula calculates the **inverse of the relative risk**, which is not what the question asks for.
- It would represent the ratio of the incidence in the unexposed group to the incidence in the exposed group.
*[(70 * 1) / (30 * 99)]*
- This is an **incorrect variation** of the odds ratio calculation, with the terms in the numerator and denominator swapped compared to the standard formula.
- Therefore, it does not represent the relative risk or a correctly calculated odds ratio.
Case-control studies US Medical PG Question 3: You are reviewing the protocol for a retrospective case-control study investigating risk factors for mesothelioma among retired factory workers. 100 cases of mesothelioma and 100 age and sex matched controls are to be recruited and interviewed about their exposure to industrial grade fiberglass by blinded interviewers. The investigators' primary hypothesis is that cases of mesothelioma will be more likely to have been exposed to industrial grade fiberglass. The design of this study is most concerning for which type of bias?
- A. This study design is free of potential bias
- B. Observer bias
- C. Interviewer bias
- D. Lead-time bias
- E. Recall bias (Correct Answer)
Case-control studies Explanation: ***Recall bias***
- In a retrospective **case-control study**, individuals with mesothelioma (cases) may be more likely to **recall and report past exposures** to industrial-grade fiberglass than controls, due to their diagnosis and their search for an explanation for their illness.
- This differential recall of past exposures between cases and controls can distort the true association between the exposure and the disease, leading to a biased estimate of risk.
- Cases do not necessarily remember more accurately; rather, they may over-report or selectively remember exposures they believe might be causally related to their disease.
*This study design is free of potential bias*
- This statement is incorrect because **no study design is completely free of potential biases**, especially in observational studies like this case-control design.
- While efforts like blinded interviewers are made, inherent limitations of retrospective data collection can introduce other forms of bias.
*Observer bias*
- **Observer bias** typically refers to situations where the researcher's expectations or beliefs influence the recording of data, but the study description states **blinded interviewers** are used, which aims to mitigate this type of bias.
- This bias is less likely here due to the blinding, and the primary concern relates to the participants' memory of past events.
*Interviewer bias*
- **Interviewer bias** can occur when the interviewer's behavior or questioning influences the participant's responses.
- However, the protocol mitigates this by using **blinded interviewers**, meaning they are unaware of the case/control status of the participants, reducing the risk of differential questioning.
*Lead-time bias*
- **Lead-time bias** is primarily a concern in screening studies where early detection of a disease might artificially prolong the survival time without actually changing the course of the disease.
- This study is investigating risk factors for mesothelioma, not evaluating the effectiveness of a screening program, rendering lead-time bias irrelevant to this design.
Case-control studies US Medical PG Question 4: Which of the following study designs would be most appropriate to investigate the association between electronic cigarette use and the subsequent development of lung cancer?
- A. Subjects with lung cancer who smoke and subjects with lung cancer who did not smoke
- B. Subjects who smoke electronic cigarettes and subjects who smoke normal cigarettes
- C. Subjects with lung cancer who smoke and subjects without lung cancer who smoke
- D. Subjects with lung cancer and subjects without lung cancer
- E. Subjects who smoke electronic cigarettes and subjects who do not smoke (Correct Answer)
Case-control studies Explanation: ***Subjects who smoke electronic cigarettes and subjects who do not smoke***
- This design represents a **cohort study**, which is ideal for investigating the **incidence** of a disease (lung cancer) in groups exposed and unexposed to a risk factor (electronic cigarette use).
- By following these two groups over time, researchers can directly compare the **risk of developing lung cancer** in e-cigarette users versus non-smokers.
*Subjects with lung cancer who smoke and subjects with lung cancer who did not smoke*
- This option incorrectly compares two groups both with lung cancer, where the exposure to smoking can either be **electronic or traditional cigarettes,** but does not provide a control group without lung cancer to assess the association.
- This design would not allow for the calculation of an **incidence rate** or a **relative risk** of lung cancer development specific to electronic cigarette use.
*Subjects who smoke electronic cigarettes and subjects who smoke normal cigarettes*
- This design compares two different types of smoking, which might be useful for comparing their relative risks but doesn't include a **non-smoking control group** to establish the absolute association with electronic cigarettes.
- While it could show if e-cigarettes are "safer" than traditional cigarettes, it wouldn't directly answer whether e-cigarettes themselves **cause lung cancer**.
*Subjects with lung cancer who smoke and subjects without lung cancer who smoke*
- This describes a **case-control study** but focuses on smoking in general rather than specifically electronic cigarettes, which is the independent variable of interest.
- While valuable for identifying risk factors, it would need to specifically differentiate between **electronic cigarette smokers** and other smokers to answer the question adequately.
*Subjects with lung cancer and subjects without lung cancer*
- This general description of a **case-control study** is too broad; it does not specify the exposure of interest, which is electronic cigarette use.
- To be relevant, the study would need to gather data on **electronic cigarette use** in both the lung cancer group and the non-lung cancer control group.
Case-control studies US Medical PG Question 5: Study X examined the relationship between coffee consumption and lung cancer. The authors of Study X retrospectively reviewed patients' reported coffee consumption and found that drinking greater than 6 cups of coffee per day was associated with an increased risk of developing lung cancer. However, Study X was criticized by the authors of Study Y. Study Y showed that increased coffee consumption was associated with smoking. What type of bias affected Study X, and what study design is geared to reduce the chance of that bias?
- A. Observer bias; double blind analysis
- B. Selection bias; randomization
- C. Lead time bias; placebo
- D. Measurement bias; blinding
- E. Confounding; randomization (Correct Answer)
Case-control studies Explanation: ***Confounding; randomization***
- Study Y suggests that **smoking** is a **confounding variable** because it is associated with both increased coffee consumption (exposure) and increased risk of lung cancer (outcome), distorting the apparent relationship between coffee and lung cancer.
- **Randomization** in experimental studies (such as randomized controlled trials) helps reduce confounding by ensuring that known and unknown confounding factors are evenly distributed among study groups.
- In observational studies where randomization is not possible, confounding can be addressed through **stratification**, **matching**, or **multivariable adjustment** during analysis.
*Observer bias; double blind analysis*
- **Observer bias** occurs when researchers' beliefs or expectations influence the study outcome, which is not the primary issue described here regarding the relationship between coffee, smoking, and lung cancer.
- **Double-blind analysis** is a method to mitigate observer bias by ensuring neither participants nor researchers know who is in the control or experimental groups.
*Selection bias; randomization*
- **Selection bias** happens when the study population is not representative of the target population, leading to inaccurate results, which is not directly indicated by the interaction between coffee and smoking.
- While **randomization** is used to reduce selection bias by creating comparable groups, the core problem identified in Study X is confounding, not flawed participant selection.
*Lead time bias; placebo*
- **Lead time bias** occurs in screening programs when early detection without improved outcomes makes survival appear longer, an issue unrelated to the described association between coffee, smoking, and lung cancer.
- A **placebo** is an inactive treatment used in clinical trials to control for psychological effects, and its relevance here is limited to treatment intervention studies.
*Measurement bias; blinding*
- **Measurement bias** arises from systematic errors in data collection, such as inaccurate patient reporting of coffee consumption, but the main criticism from Study Y points to a third variable (smoking) affecting the association, not just flawed measurement.
- **Blinding** helps reduce measurement bias by preventing participants or researchers from knowing group assignments, thus minimizing conscious or unconscious influences on data collection.
Case-control studies US Medical PG Question 6: A medical research study is beginning to evaluate the positive predictive value of a novel blood test for non-Hodgkin’s lymphoma. The diagnostic arm contains 700 patients with NHL, of which 400 tested positive for the novel blood test. In the control arm, 700 age-matched control patients are enrolled and 0 are found positive for the novel test. What is the PPV of this test?
- A. 400 / (400 + 0) (Correct Answer)
- B. 700 / (700 + 300)
- C. 400 / (400 + 300)
- D. 700 / (700 + 0)
- E. 700 / (400 + 400)
Case-control studies Explanation: ***400 / (400 + 0) = 1.0 or 100%***
- The **positive predictive value (PPV)** is calculated as **True Positives / (True Positives + False Positives)**.
- In this scenario, **True Positives (TP)** are the 400 patients with NHL who tested positive, and **False Positives (FP)** are 0, as no control patients tested positive.
- This gives a PPV of 400/400 = **1.0 or 100%**, indicating that all patients who tested positive actually had the disease.
*700 / (700 + 300)*
- This calculation does not align with the formula for PPV based on the given data.
- The denominator `(700+300)` suggests an incorrect combination of various patient groups.
*400 / (400 + 300)*
- The denominator `(400+300)` incorrectly includes 300, which is the number of **False Negatives** (patients with NHL who tested negative), not False Positives.
- PPV focuses on the proportion of true positives among all positive tests, not all diseased individuals.
*700 / (700 + 0)*
- This calculation incorrectly uses the total number of patients with NHL (700) as the numerator, rather than the number of positive test results in that group.
- The numerator should be the **True Positives** (400), not the total number of diseased individuals.
*700 / (400 + 400)*
- This calculation uses incorrect values for both the numerator and denominator, not corresponding to the PPV formula.
- The numerator 700 represents the total number of patients with the disease, not those who tested positive, and the denominator incorrectly sums up values that don't represent the proper PPV calculation.
Case-control studies US Medical PG Question 7: You are currently employed as a clinical researcher working on clinical trials of a new drug to be used for the treatment of Parkinson's disease. Currently, you have already determined the safe clinical dose of the drug in a healthy patient. You are in the phase of drug development where the drug is studied in patients with the target disease to determine its efficacy. Which of the following phases is this new drug currently in?
- A. Phase 4
- B. Phase 1
- C. Phase 2 (Correct Answer)
- D. Phase 0
- E. Phase 3
Case-control studies Explanation: ***Phase 2***
- **Phase 2 trials** involve studying the drug in patients with the target disease to assess its **efficacy** and further evaluate safety, typically involving a few hundred patients.
- The question describes a stage after safe dosing in healthy patients (Phase 1) and before large-scale efficacy confirmation (Phase 3), focusing on efficacy in the target population.
*Phase 4*
- **Phase 4 trials** occur **after a drug has been approved** and marketed, monitoring long-term effects, optimal use, and rare side effects in a diverse patient population.
- This phase is conducted post-market approval, whereas the question describes a drug still in development prior to approval.
*Phase 1*
- **Phase 1 trials** primarily focus on determining the **safety and dosage** of a new drug in a **small group of healthy volunteers** (or sometimes patients with advanced disease if the drug is highly toxic).
- The question states that the safe clinical dose in a healthy patient has already been determined, indicating that Phase 1 has been completed.
*Phase 0*
- **Phase 0 trials** are exploratory, very early-stage studies designed to confirm that the drug reaches the target and acts as intended, typically involving a very small number of doses and participants.
- These trials are conducted much earlier in the development process, preceding the determination of safe clinical doses and large-scale efficacy studies.
*Phase 3*
- **Phase 3 trials** are large-scale studies involving hundreds to thousands of patients to confirm **efficacy**, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
- While Phase 3 does assess efficacy, it follows Phase 2 and is typically conducted on a much larger scale before submitting for regulatory approval.
Case-control studies US Medical PG Question 8: A new study is investigating the effects of an experimental drug, Exerzisin, on the duration and intensity of exercise. In the treatment group participants are given daily Exerzisin at the main treatment facility and instructed to exercise as much as they would like on the facility's exercise equipment. Due to an insufficient number of exercise units at the main treatment center, the control subjects are given free access to an outside, private gym. The duration and intensity of exercise in both groups is measured with a pedometer. The perspicacious undergraduate, hired to input all the data, points out that the treatment group may be more motivated to exercise harder and longer because their exercising can be observed by the investigators. To which form of bias is he alluding?
- A. Selection bias
- B. Recall bias
- C. Hawthorne effect (Correct Answer)
- D. Pygmalion effect
- E. Lead time bias
Case-control studies Explanation: ***Hawthorne effect***
- The Hawthorne effect describes the phenomenon where individuals modify an aspect of their behavior in response to their awareness of being **observed**.
- In this study, the treatment group may exercise more intensely because they know investigators are watching, potentially confounding the drug's true effect.
*Selection bias*
- **Selection bias** occurs when the selection of subjects for a study, or their likelihood of staying in the study, leads to a sample that does not accurately represent the target population.
- While there are issues with how the control and treatment groups are handled, the specific concern here is about behavior change due to observation, not how initial subjects were chosen or lost.
*Recall bias*
- **Recall bias** is a systematic error caused by differences in the accuracy or completeness of the recollections of past events or experiences by study participants.
- This type of bias is relevant when participants are asked to remember past information, which is not the primary issue in this scenario where current exercise behavior is being observed.
*Pygmalion effect*
- The **Pygmalion effect** occurs when higher expectations placed upon individuals lead to better performance.
- This typically involves an investigator's expectations influencing a participant's performance, rather than the participant's awareness of being observed influencing their own behavior.
*Lead time bias*
- **Lead time bias** is a form of bias that occurs in screening and early detection studies, where earlier diagnosis of a disease (due to screening) makes it seem like patients live longer, even if the treatment doesn't change the actual course of the disease.
- This bias is not relevant here as the study is observing the effects of a drug on exercise, not disease prognosis or survival.
Case-control studies US Medical PG Question 9: Researchers are studying the relationship between heart disease and alcohol consumption. They review the electronic medical records of 500 patients at a local hospital during the study period and identify the presence or absence of acute coronary syndrome (ACS) and the number of alcoholic drinks consumed on the day of presentation. They find that there is a lower prevalence of acute coronary syndrome in patients who reported no alcohol consumption or 1 drink daily compared with those who reported 2 or more drinks. Which of the following is the most accurate description of this study type?
- A. Cross-sectional study
- B. Prospective study
- C. Randomized controlled trial
- D. Case-control study
- E. Retrospective study (Correct Answer)
Case-control studies Explanation: ***Retrospective study***
- This study **reviews electronic medical records** that were created in the past, making it retrospective by definition.
- Researchers looked **backward in time** during the study period to identify both the exposure (alcohol consumption) and outcome (ACS) from existing records.
- The key feature is that **data collection relies on pre-existing documentation** rather than prospectively following patients or collecting data at a single point in time.
- This is specifically a **retrospective cohort design** where researchers identified a population and assessed both exposure and outcome from historical records.
*Cross-sectional study*
- Cross-sectional studies collect data from participants at a **single point in time** through surveys, interviews, or direct assessment—not by reviewing past medical records.
- While this study assessed variables "at presentation," the **method of data collection** (reviewing electronic records retrospectively) makes it retrospective, not cross-sectional.
- Cross-sectional studies typically involve **active data collection** from living participants, not record review.
*Prospective study*
- A prospective study follows participants **forward in time** from exposure to outcome, recruiting them before outcomes develop.
- This study did not follow patients forward; it reviewed **records of events that already occurred**.
*Randomized controlled trial*
- An RCT involves **intervention and randomization** of participants to different treatment groups.
- This is an observational study with no intervention or randomization.
*Case-control study*
- A case-control study first identifies **cases (with disease)** and **controls (without disease)**, then looks backward to compare exposures.
- This study did not select participants based on disease status first; it reviewed a general hospital population and assessed both variables simultaneously from records.
Case-control studies US Medical PG Question 10: A 16-year-old girl comes to the physician because she is worried about gaining weight. She reports that at least twice a week, she eats excessive amounts of food but feels ashamed about losing control soon after. She is very active in her high school's tennis team and goes running daily to lose weight. She has a history of cutting her forearms with the metal tab from a soda can. Her last menstrual period was 3 weeks ago. She is 165 cm (5 ft 5 in) tall and weighs 57 kg (125 lb); BMI is 21 kg/m2. Physical examination shows enlarged, firm parotid glands bilaterally. There are erosions of the enamel on the lingual surfaces of the teeth. Which of the following is the most likely diagnosis?
- A. Borderline personality disorder
- B. Bulimia nervosa (Correct Answer)
- C. Body dysmorphic disorder
- D. Anorexia nervosa
- E. Obsessive-compulsive disorder
Case-control studies Explanation: ***Bulimia nervosa***
- This patient exhibits characteristic features of bulimia nervosa, including recurrent episodes of **binge eating** (at least twice weekly) followed by inappropriate **compensatory behaviors**.
- The **bilateral parotid gland enlargement** and **lingual enamel erosion** are **pathognomonic physical signs of chronic self-induced vomiting** (purging behavior), combined with excessive exercise as additional compensation.
- Her normal BMI of 21 kg/m² is highly consistent with bulimia nervosa, as individuals with this condition typically maintain a **normal weight or are overweight**, unlike those with anorexia nervosa.
- The sense of **loss of control** and **shame** about eating episodes are core features of this disorder.
*Borderline personality disorder*
- While **self-harm** (cutting) can be associated with borderline personality disorder, the primary concern in this patient is the prominent eating disorder symptoms with pathognomonic physical findings.
- Borderline personality disorder is characterized by a pervasive pattern of **instability in interpersonal relationships**, self-image, affects, and marked impulsivity; these are not the main presenting complaints here.
- Self-harm behavior can occur in multiple psychiatric conditions and does not alone establish this diagnosis.
*Body dysmorphic disorder*
- This disorder involves a **preoccupation with perceived flaws in physical appearance** that are minimal or unobservable to others, leading to significant distress or impairment.
- While the patient is concerned about gaining weight, her primary symptoms revolve around **binge-purge cycles** with physical evidence of purging behavior, rather than a sole preoccupation with a specific body defect.
*Anorexia nervosa*
- Anorexia nervosa is characterized by **restriction of energy intake** leading to a significantly low body weight (BMI usually <17.5 kg/m²) and intense fear of gaining weight despite being underweight.
- This patient has a **normal BMI (21 kg/m²)** and engages in binge-eating followed by compensatory behaviors (purging and exercise), which represents bulimia nervosa rather than anorexia nervosa.
- Additionally, she has **regular menses** (last period 3 weeks ago), whereas amenorrhea is common in anorexia nervosa due to low body weight.
*Obsessive-compulsive disorder*
- OCD involves recurrent, persistent, **intrusive thoughts (obsessions)** and/or repetitive behaviors or mental acts that an individual feels driven to perform **(compulsions)** to reduce anxiety.
- While some of the patient's behaviors might seem ritualistic, the core symptoms are clearly related to **eating disorder pathology with binge-purge cycles**, not typical OCD themes like contamination, symmetry, or checking behaviors.
- The physical signs of chronic purging behavior definitively point to an eating disorder diagnosis.
More Case-control studies US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.