Cardiac looping US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Cardiac looping. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
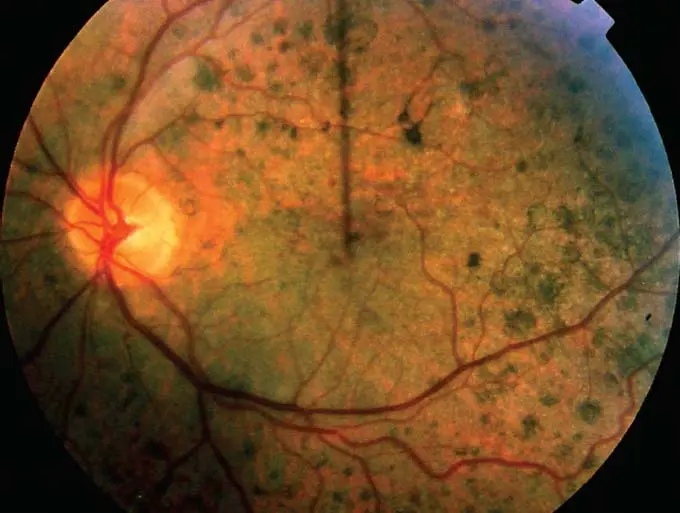
Cardiac looping US Medical PG Question 1: A male neonate is being examined by a pediatrician. His mother informs the doctor that she had a mild fever with rash, muscle pain, and swollen and tender lymph nodes during the second month of gestation. The boy was born at 39 weeks gestation via spontaneous vaginal delivery with no prenatal care. On physical examination, the neonate has normal vital signs. Retinal examination reveals the findings shown in the image. Which of the following congenital heart defects is most likely to be present in this neonate?
- A. Double outlet right ventricle
- B. Atrial septal defect
- C. Patent ductus arteriosus (Correct Answer)
- D. Ventricular septal defect
- E. Tetralogy of Fallot
Cardiac looping Explanation: ***Patent ductus arteriosus***
- This neonate has **congenital rubella syndrome (CRS)** based on maternal symptoms during the first trimester (fever, rash, lymphadenopathy) and the characteristic **"salt and pepper" retinopathy** shown on retinal examination
- **PDA is the most common cardiac defect** associated with CRS, occurring in approximately 50-85% of affected infants
- Other cardiac manifestations of CRS include peripheral pulmonary artery stenosis, but PDA predominates
- The classic triad of CRS includes cardiac defects, ocular abnormalities (cataracts, glaucoma, retinopathy), and sensorineural deafness
*Double outlet right ventricle*
- This is a **conotruncal anomaly** typically presenting with cyanosis in the neonatal period
- Not associated with maternal rubella infection or congenital rubella syndrome
- Would present with abnormal ventricular anatomy and significant hemodynamic compromise
*Atrial septal defect*
- While ASD is a common congenital heart defect, it is **not characteristically associated with CRS**
- Much less frequently linked to maternal viral infections compared to PDA
- Often asymptomatic in the neonatal period and detected later in childhood
*Ventricular septal defect*
- VSD is less commonly associated with **congenital rubella syndrome** compared to PDA
- When present, typically manifests with a holosystolic murmur at the left lower sternal border
- Can occur with maternal infections but is not the predominant cardiac finding in CRS
*Tetralogy of Fallot*
- Consists of four anatomic abnormalities: VSD, pulmonary stenosis, overriding aorta, and right ventricular hypertrophy
- Presents with **cyanosis** ("tet spells") and is not specifically linked to maternal rubella infection
- Not part of the congenital rubella syndrome spectrum
Cardiac looping US Medical PG Question 2: Cardiac muscle serves many necessary functions, leading to a specific structure that serves these functions. The structure highlighted is an important histology component of cardiac muscle. What would be the outcome if this structure diffusely failed to function?
- A. Failure of potassium channels to appropriately open to repolarize the cell
- B. Failure of propagation of the action potential from the conduction system (Correct Answer)
- C. Ineffective excitation-contraction coupling due to insufficient calcium ions
- D. Inappropriate formation of cardiac valve leaflets
- E. Outflow tract obstruction
Cardiac looping Explanation: ***Failure of propagation of the action potential from the conduction system***
- The highlighted structure, the **intercalated disc**, contains **gap junctions** which are crucial for the rapid, synchronized spread of **action potentials** between cardiac muscle cells.
- A diffuse failure of these structures would prevent the coordinated electrical activation of the myocardium, leading to a failure of impulse propagation and **compromised cardiac contraction**.
*Failure of potassium channels to appropriately open to repolarize the cell*
- This scenario describes a problem with **ion channel function** within individual cardiomyocytes, affecting their repolarization phase.
- While critical for a single cell's electrical activity, it does not directly relate to the primary function of **intercalated discs** in *propagating* action potentials across multiple cells.
*Ineffective excitation-contraction coupling due to insufficient calcium ions*
- This outcome would result from issues with **calcium handling** mechanisms, such as problems with the **sarcoplasmic reticulum** or **calcium channels**, which are internal to the cardiomyocyte.
- It is distinct from the role of **intercalated discs** in facilitating intercellular communication and electrical spread.
*Inappropriate formation of cardiac valve leaflets*
- The formation of cardiac valve leaflets is an intricate process during **embryological development** involving specific signaling pathways and cell migration.
- This structural defect is not directly related to the function of **intercalated discs** in mature cardiac muscle, which are involved in electrical and mechanical coupling.
*Outflow tract obstruction*
- **Outflow tract obstruction** is a congenital or acquired structural defect affecting the major arteries leaving the heart (e.g., aortic or pulmonary stenosis).
- This is a macroscopic structural anomaly that is not caused by a primary failure of **intercalated disc** function.
Cardiac looping US Medical PG Question 3: A 4-year-old male is brought into your office because his mother states he has been fatigued. He has not been acting like himself and has been getting tired easily while running around and playing with other children. As of last week, he has also been complaining of being short of breath. His vitals are temperature 98.6 deg F (37.2 deg C), blood pressure 100/75 mmHg, pulse 98/min, and respirations 22/min. On exam, the patient is short of breath, and there is a holosystolic murmur with an appreciable thrill along the left sternal border. There are no other noticeable abnormalities, and the mother states that the child's prenatal course along with genetic testing was normal. What is the most likely diagnosis?
- A. Atrial septal defect (ASD)
- B. Endocardial cushion defect
- C. Ventricular septal defect (VSD) (Correct Answer)
- D. Patent ductus arteriosus (PDA)
- E. Tetralogy of Fallot
Cardiac looping Explanation: ***Ventricular septal defect (VSD)***
- A **holosystolic murmur** at the **left sternal border** with an associated **thrill** is a classic finding for a VSD.
- The patient's fatigue and shortness of breath (evidencing activity intolerance) are signs of **left-to-right shunting** causing increased pulmonary blood flow and eventual heart failure.
*Atrial septal defect (ASD)*
- An ASD typically presents with a **systolic ejection murmur** at the upper left sternal border due to increased flow across the pulmonic valve, not a holosystolic murmur.
- While it can cause fatigue and dyspnea, a thrill is less common, and the murmur quality is distinct.
*Endocardial cushion defect*
- This defect involves the atrioventricular septum, often resulting in a **split S2** and a **holosystolic murmur** best heard at the lower left sternal border.
- However, it is strongly associated with **Down syndrome**, which is ruled out by the normal genetic testing.
*Patent ductus arteriosus (PDA)*
- PDA is characterized by a **continuous "machinery" murmur** best heard below the left clavicle, which is distinct from the holosystolic murmur described.
- The associated symptoms of fatigue and dyspnea can occur, but the murmur differentiates it.
*Tetralogy of Fallot*
- This condition presents with **cyanosis** and a **crescendo-decrescendo systolic ejection murmur** at the left sternal border, not a holosystolic murmur with a thrill.
- Patients often exhibit "tet spells" and **clubbing**, which are absent in this presentation.
Cardiac looping US Medical PG Question 4: A child is in the nursery one day after birth. A nurse notices a urine-like discharge being expressed through the umbilical stump. What two structures in the embryo are connected by the structure that failed to obliterate during the embryologic development of this child?
- A. Kidney - large bowel
- B. Liver - umbilical vein
- C. Bladder - small bowel
- D. Pulmonary artery - aorta
- E. Bladder - umbilicus (Correct Answer)
Cardiac looping Explanation: ***Bladder - umbilicus***
- A **urine-like discharge** from the umbilical stump indicates a **patent urachus**, which is the embryonic remnant of the allantois.
- The **allantois** (which becomes the urachus) is an embryonic structure that connects the **fetal bladder** to the **umbilicus** during development.
- Normally, the allantois obliterates after birth to form the **median umbilical ligament**, but failure to obliterate results in a patent urachus allowing urine to discharge through the umbilicus.
*Kidney - large bowel*
- These two structures are not directly connected by an obliterating embryonic structure relevant to urine discharge from an umbilical stump.
- The kidneys form urine, and the large bowel is part of the digestive tract, with no direct embryonic communication to the umbilicus for urine expression.
*Liver - umbilical vein*
- The umbilical vein connects the **placenta to the fetal liver** (and ductus venosus) to transport oxygenated blood, not urine.
- Failure of the umbilical vein to obliterate would result in a patent umbilical vein, typically presenting as a vascular anomaly, not urine discharge.
*Pulmonary artery - aorta*
- These structures are connected by the **ductus arteriosus** in fetal circulation, bypassing the pulmonary circulation.
- While important for fetal development, a patent ductus arteriosus (PDA) is a cardiovascular anomaly and would not manifest as urine discharge from the umbilical stump.
*Bladder - small bowel*
- While both structures are involved in waste elimination, there is no normal embryonic structure directly connecting the bladder and small bowel that obliterates to prevent urine discharge from the umbilicus.
- An abnormal connection between the bladder and bowel would typically involve a **fistula** and present with stool in urine or urine in stool, not umbilical discharge.
Cardiac looping US Medical PG Question 5: A 26-year-old woman comes to the physician for evaluation of nausea and fatigue. Her last menstrual period was 8 weeks ago. She has a history of bipolar disorder controlled by a drug known to sometimes cause hypothyroidism and nephrogenic diabetes insipidus. She does not smoke cigarettes or drink alcohol. A urine pregnancy test is positive. An ultrasound of the pelvis shows a viable intrauterine pregnancy. The fetus is most likely at increased risk for which of the following anomalies?
- A. Neural tube defects
- B. Aplasia cutis
- C. Hypoplastic or absent limbs
- D. Abnormal placentation
- E. Atrialization of the right ventricle (Correct Answer)
Cardiac looping Explanation: ***Atrialization of the right ventricle***
- The patient's history of **bipolar disorder** controlled by a drug causing **hypothyroidism** and **nephrogenic diabetes insipidus** strongly points to **lithium**.
- **Lithium** exposure during the first trimester of pregnancy is associated with an increased risk of **Ebstein's anomaly**, which involves the **apical displacement of the tricuspid valve** leaflets leading to **atrialization of the right ventricle**.
*Neural tube defects*
- These anomalies are often associated with deficiencies in **folic acid** or exposure to certain **antiepileptic drugs** like valproate, not lithium.
- While concerning, there is no information in the vignette to suggest these specific risk factors exist for this patient besides lithium use.
*Aplasia cutis*
- This is a localized absence of skin at birth, most commonly on the scalp. It is associated with gestational exposure to **methimazole** or **carbimazole**, used to treat hyperthyroidism, which is not indicated here.
- There is no direct link between lithium exposure and aplasia cutis.
*Hypoplastic or absent limbs*
- This type of anomaly is historically associated with exposure to **thalidomide** during early pregnancy.
- Lithium is not known to cause limb reduction defects.
*Abnormal placentation*
- Conditions like **placenta previa** or **placenta accreta** can result from previous uterine surgery (e.g., C-section) or advanced maternal age.
- Lithium use is not a recognized risk factor for abnormal placentation.
Cardiac looping US Medical PG Question 6: Shortly after delivery, a female newborn develops bluish discoloration of the lips, fingers, and toes. She was born at term to a 38-year-old primigravid woman. Pregnancy was complicated by maternal diabetes mellitus. Pulse oximetry on room air shows an oxygen saturation of 81%. Echocardiography shows immediate bifurcation of the vessel arising from the left ventricle; the vessel emerging from the right ventricle gives out coronary, head, and neck vessels. An abnormality in which of the following developmental processes most likely accounts for this patient's condition?
- A. Alignment of infundibular septum
- B. Fusion of endocardial cushion
- C. Spiraling of aorticopulmonary septum (Correct Answer)
- D. Separation of tricuspid valve tissue from myocardium
- E. Division of aorta and pulmonary artery
Cardiac looping Explanation: ***Spiraling of aorticopulmonary septum***
- The description of a vessel arising from the left ventricle that immediately bifurcates (pulmonary artery) and a vessel from the right ventricle that gives off coronary, head, and neck vessels (aorta) is characteristic of **transposition of the great arteries (TGA)**.
- TGA results from a failure of the **aorticopulmonary septum** to spiral during embryological development, leading to the aorta originating from the right ventricle and the pulmonary artery from the left ventricle.
*Alignment of infundibular septum*
- Abnormal alignment of the infundibular septum is associated with conditions like **tetralogy of Fallot**, which involves a maligned interventricular septum and a shifted aorta, presenting with a different set of echocardiographic findings.
- While also a **cyanotic heart defect**, tetralogy of Fallot's anatomy (e.g., interventricular septal defect, pulmonary stenosis) differs from the described TGA.
*Fusion of endocardial cushion*
- Failure of fusion of the **endocardial cushions** leads to **atrioventricular septal defects**, which involve defects in both the atria and ventricular septa, and often affect the mitral and tricuspid valves.
- These defects typically present with heart failure symptoms and different echocardiographic findings than those described for TGA.
*Separation of tricuspid valve tissue from myocardium*
- An abnormality in the separation of tricuspid valve tissue from the myocardium is the cause of **Ebstein anomaly**, where the tricuspid valve leaflets are displaced downwards into the right ventricle, leading to tricuspid regurgitation.
- Ebstein anomaly is characterized by right atrial enlargement and a largely functional right ventricle, leading to issues with right heart output but not the great artery transposition described.
*Division of aorta and pulmonary artery*
- The division of the truncus arteriosus into the aorta and pulmonary artery is a normal developmental process, which when complete usually produces the correct great artery connections. However, the exact arrangement of these vessels is determined by the **spiraling of the aorticopulmonary septum**, not just the division itself.
- Failure of this division, resulting in a **persistent truncus arteriosus**, would present as a single great artery arising from both ventricles, which is distinct from the two separate but transposed vessels seen in TGA.
Cardiac looping US Medical PG Question 7: A newborn is rushed to the neonatal ICU after becoming cyanotic shortly after birth. An ultrasound is performed which shows the aorta coming off the right ventricle and lying anterior to the pulmonary artery. The newborn is given prostaglandin E1 and surgery is planned to correct the anatomic defect. Which of the following developmental processes failed to occur in the newborn?
- A. Failure of the membranous ventricular septum to fuse with the muscular interventricular septum
- B. Failure of the septum primum to fuse with the septum secundum
- C. Failure of the aorticopulmonary septum to spiral (Correct Answer)
- D. Failure of the ductus venosus to close
- E. Failure of the ductus arteriosus to close
Cardiac looping Explanation: ***Failure of the aorticopulmonary septum to spiral***
- **Transposition of the great arteries (TGA)**, characterized by the aorta originating from the right ventricle and the pulmonary artery from the left ventricle, results from the **aorticopulmonary septum** failing to spiral properly during embryological development.
- This defect leads to two separate circulatory systems, causing severe **cyanosis** shortly after birth and requiring **prostaglandin E1** to maintain a patent ductus arteriosus for mixing of oxygenated and deoxygenated blood.
- This is a ductal-dependent lesion requiring urgent intervention.
*Failure of the membranous ventricular septum to fuse with the muscular interventricular septum*
- This specific failure leads to a **ventricular septal defect (VSD)**, which allows blood to shunt between ventricles.
- While VSDs can cause cyanosis if large and associated with pulmonary hypertension (Eisenmenger syndrome), the description of **great artery transposition** is not caused by this developmental failure.
*Failure of the septum primum to fuse with the septum secundum*
- This developmental anomaly results in a **patent foramen ovale (PFO)** or an **atrial septal defect (ASD)**.
- These defects typically cause a left-to-right shunt and present with symptoms later in life, not with severe immediate cyanosis.
- In TGA, an ASD may actually be beneficial as it allows some mixing of blood.
*Failure of the ductus venosus to close*
- The **ductus venosus** shunts oxygenated blood from the umbilical vein directly to the inferior vena cava, bypassing the fetal liver during intrauterine life.
- Persistent patency of the ductus venosus after birth is rare and does not cause the severe cyanosis and specific great artery anatomy seen in TGA.
*Failure of the ductus arteriosus to close*
- A **patent ductus arteriosus (PDA)** allows blood to flow from the aorta to the pulmonary artery after birth, which can lead to pulmonary overcirculation.
- In **transposition of the great arteries**, a PDA is actually crucial for survival as it provides a pathway for mixing of oxygenated and deoxygenated blood; maintaining PDA patency with PGE1 is the initial management, not a cause of the condition.
Cardiac looping US Medical PG Question 8: A 28-year-old woman with corrected transposition of the great arteries (L-TGA) who has been asymptomatic presents for preconception counseling. She has a systemic right ventricle supporting systemic circulation and asks about pregnancy risks. Her cardiologist notes mild tricuspid regurgitation. Evaluate the embryologic basis of her condition and synthesize recommendations regarding pregnancy.
- A. Simple transposition with late correction; pregnancy is safe with standard monitoring
- B. Both AV and ventriculoarterial discordance creating physiologically corrected circulation; pregnancy acceptable if systemic RV function normal, but requires high-risk obstetric and cardiology co-management (Correct Answer)
- C. Uncorrected transposition incompatible with pregnancy; recommend adoption
- D. Iatrogenic correction; pregnancy safe as anatomy is normalized
- E. Partial transposition; standard prenatal care is sufficient
Cardiac looping Explanation: ***Both AV and ventriculoarterial discordance creating physiologically corrected circulation; pregnancy acceptable if systemic RV function normal, but requires high-risk obstetric and cardiology co-management***
- **L-TGA** involves **levo-looping** of the heart tube where the **morphologic right ventricle** (RV) supports the systemic circulation due to double discordance (atrioventricular and ventriculoarterial).
- Pregnancy is generally tolerated (maternal WHO class III) if **systemic RV function** is preserved, but requires multidisciplinary care to monitor for **heart failure**, **arrhythmias**, and worsening **tricuspid regurgitation**.
*Simple transposition with late correction; pregnancy is safe with standard monitoring*
- **D-TGA** (simple transposition) requires surgical correction (e.g., Arterial Switch) and has a distinct embryology involving failure of **conotruncal septation** spiral.
- Unlike L-TGA, corrected D-TGA carries different risks and would not be classified as having a "systemic right ventricle" if an **arterial switch** was performed.
*Uncorrected transposition incompatible with pregnancy; recommend adoption*
- **L-TGA** is "congenitally corrected," meaning blood flows in the correct physiological sequence; it is not inherently incompatible with pregnancy if the **systemic RV** is functional.
- Maternal mortality is not high enough to warrant absolute contraindication unless there is severe **RV dysfunction** or NYHA Class III/IV symptoms.
*Iatrogenic correction; pregnancy safe as anatomy is normalized*
- This condition is **congenitally corrected**, meaning the "correction" occurred during **embryogenesis** due to the double mismatch, not through surgery.
- The anatomy is never truly "normalized" because the **tricuspid valve** and **RV** are not designed for high-pressure systemic resistance, making pregnancy a high-risk event.
*Partial transposition; standard prenatal care is sufficient*
- There is no clinical entity termed "partial transposition" in this context; L-TGA is a complete, albeit **physiologically corrected**, malformation.
- Standard prenatal care is insufficient because the hemodynamic stress of pregnancy can trigger **systemic RV failure** or significant **heart block**.
Cardiac looping US Medical PG Question 9: A newborn presents with severe cyanosis, hypoplastic right ventricle, pulmonary atresia, and an intact ventricular septum. The cardiologist notes this differs from tetralogy of Fallot despite both having pulmonary atresia. The neonatologist questions whether to maintain ductal patency or pursue immediate surgical intervention. Evaluate the embryologic differences and synthesize the optimal management strategy.
- A. Both result from infundibular deviation; maintain ductus with prostaglandins and perform staged repair
- B. Pulmonary atresia with intact septum results from primary valve failure; urgent surgical valvotomy or perforation
- C. Both are conotruncal defects; immediate complete repair is preferred
- D. Pulmonary atresia with intact septum results from primary valve failure; maintain ductus and evaluate RV-dependent coronary circulation before intervention (Correct Answer)
- E. Different embryologic timing but similar management; prostaglandin with elective repair at 6 months
Cardiac looping Explanation: ***Pulmonary atresia with intact septum results from primary valve failure; maintain ductus and evaluate RV-dependent coronary circulation before intervention***
- Unlike Tetralogy of Fallot which is a **conotruncal defect**, Pulmonary Atresia with Intact Ventricular Septum (PA-IVS) is a primary **valvular failure** resulting in secondary right ventricular hypoplasia.
- It is critical to identify **RV-dependent coronary circulation** via sinusoids before any intervention, as decompressing the right ventricle in these patients can lead to fatal **myocardial ischemia**.
*Both result from infundibular deviation; maintain ductus with prostaglandins and perform staged repair*
- Only Tetralogy of Fallot results from the **anterior deviation** of the infundibular septum, whereas PA-IVS is characterized by a lack of communication between the RV and pulmonary artery without a VSD.
- While prostaglandins are used in both for **ductal patency**, the underlying embryologic mechanism and surgical risks differ significantly.
*Pulmonary atresia with intact septum results from primary valve failure; urgent surgical valvotomy or perforation*
- Although it is a primary valve failure, **urgent valvotomy** is contraindicated if there is **RV-dependent coronary circulation**.
- Releasing the high pressure in the RV can cause reversal of flow in **coronary sinusoids**, leading to sudden cardiac death.
*Both are conotruncal defects; immediate complete repair is preferred*
- PA-IVS is not a **conotruncal defect** because it lacks the malalignment of the outflow tract septum typically seen in Tetralogy or Transposition.
- **Complete repair** is often impossible in the neonatal period due to the **hypoplastic right ventricle** and anatomical constraints.
*Different embryologic timing but similar management; prostaglandin with elective repair at 6 months*
- Management is not similar because PA-IVS requires immediate mapping of the **coronary arteries** and high risk of **RV-dependent** flow.
- Elective repair at 6 months is often too late for stable management; these neonates require **prostaglandin (PGE1)** and early palliative or corrective intervention within the first weeks of life.
Cardiac looping US Medical PG Question 10: A pregnant woman at 18 weeks gestation with poorly controlled phenylketonuria undergoes fetal echocardiography showing coarctation of the aorta and a VSD. Her obstetrician is concerned about the timing of teratogenic exposure. Evaluate the critical periods of cardiac development to determine when the damage likely occurred and synthesize a management approach.
- A. Week 12-16; defects likely due to recent metabolic derangement, aggressive dietary intervention may reverse changes
- B. Week 2-3; recommend immediate dietary modification and serial echocardiograms
- C. Week 3-8; damage already occurred, focus on optimizing current metabolic control and pediatric cardiology consultation (Correct Answer)
- D. Week 8-12; current dietary control may prevent progression, increase monitoring frequency
- E. Throughout pregnancy; implement strict dietary control and consider early delivery
Cardiac looping Explanation: ***Week 3-8; damage already occurred, focus on optimizing current metabolic control and pediatric cardiology consultation***
- The **embryonic period** (weeks 3 to 8) is the critical window for **organogenesis**, including the development of the cardiac septa and outflow tracts.
- Structural defects like **coarctation of the aorta** and **VSD** originate during this window, meaning the damage is irreversible; management must transition to **postnatal planning** and stabilization of phenylalanine levels.
*Week 12-16; defects likely due to recent metabolic derangement, aggressive dietary intervention may reverse changes*
- By the second trimester, the **structural anatomy** of the heart is already established, so metabolic insults at this stage do not cause primary structural defects.
- **Dietary intervention** cannot reverse anatomical malformations like septal or aortic wall defects once they have formed.
*Week 2-3; recommend immediate dietary modification and serial echocardiograms*
- While the **heart tube** begins to form late in week 3, the complex development of the chambers and septa occurs primarily after this short window.
- High phenylalanine levels at week 2 might lead to early pregnancy loss, but **teratogenic malformations** are classically associated with the later organogenesis period.
*Week 8-12; current dietary control may prevent progression, increase monitoring frequency*
- By week 8, the **cardiac septation** process is largely complete, and major anatomical structures are already localized.
- **Prevention of progression** is not applicable to structural anomalies like a VSD, which represent a failure of tissue fusion rather than a progressive disease state.
*Throughout pregnancy; implement strict dietary control and consider early delivery*
- While phenylalanine affects the fetus throughout pregnancy, structural **congenital heart disease** is specifically linked to tissues differentiating in the first trimester.
- **Early delivery** at 18 weeks is not a viable management strategy for fetal cardiac defects and would result in extreme prematurity.
More Cardiac looping US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.