Pancreas development US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Pancreas development. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Pancreas development US Medical PG Question 1: A new mother expresses her concerns because her 1-day-old newborn has been having feeding difficulties. The child vomits after every feeding and has had a continuous cough since shortly after birth. The mother denies any greenish coloration of the vomit and says that it is only composed of whitish milk that the baby just had. The child exhibits these coughing spells during the exam, at which time the physician notices the child’s skin becoming cyanotic. The mother states that the child was born vaginally with no complications, although her records show that she had polyhydramnios during her last ultrasound before the delivery. Which of the following is the most likely cause of the patient’s symptoms?
- A. Failure of recanalization of duodenum
- B. Obstruction due to failure of rotation of pancreatic tissue
- C. Hypertrophy of the pyloric sphincter
- D. Failure of neural crest cells to migrate into the myenteric plexus
- E. Defective formation of the esophagus with tracheoesophageal connection (Correct Answer)
Pancreas development Explanation: ***Defective formation of the esophagus with tracheoesophageal connection***
- The combination of **feeding difficulties**, vomiting of **undigested milk**, **continuous coughing**, and **cyanosis** during coughing spells in a newborn strongly suggests a **tracheoesophageal fistula (TEF)**, often with **esophageal atresia**.
- **Polyhydramnios** during pregnancy is a classic prenatal sign due to the fetal inability to swallow amniotic fluid.
*Failure of recanalization of duodenum*
- This condition, known as **duodenal atresia**, typically presents with **bilious vomiting** if the obstruction is distal to the ampulla of Vater, or non-bilious if proximal, but typically does not cause continuous coughing or cyanosis during feeds.
- While it causes vomiting, it primarily affects digestion and nutrient absorption, and **polyhydramnios** can also be present due to impaired fetal swallowing.
*Obstruction due to failure of rotation of pancreatic tissue*
- This describes **annular pancreas**, where pancreatic tissue encircles the duodenum, causing obstruction and **vomiting** (often bilious).
- Like duodenal atresia, it doesn't explain the characteristic **coughing and cyanosis** with feeds seen in this case.
*Hypertrophy of the pyloric sphincter*
- **Pyloric stenosis** typically presents later (2-8 weeks of age) with **projectile non-bilious vomiting**, and an **olive-shaped mass** may be palpable in the abdomen.
- It does not cause coughing or cyanosis that correlates directly with feeding in a 1-day-old.
*Failure of neural crest cells to migrate into the myenteric plexus*
- This describes **Hirschsprung disease**, which primarily affects the colon and presents with symptoms of **intestinal obstruction** (e.g., abdominal distension, failure to pass meconium, bilious vomiting if severe).
- It is not associated with feeding difficulties, coughing, or cyanosis in the manner described.
Pancreas development US Medical PG Question 2: A 4-week-old infant is brought to the emergency department by his parents with violent vomiting. It started about 3 days ago and has slowly gotten worse. He vomits after most feedings but seems to keep some formula down. His mother notes that he is eager to feed between episodes and seems to be putting on weight. Other than an uncomplicated course of chlamydia conjunctivitis, the infant has been healthy. He was born at 39 weeks gestation via spontaneous vaginal delivery. He is up to date on all vaccines and is meeting all developmental milestones. The physical exam is significant for a palpable mass in the right upper quadrant. What is the first-line confirmatory diagnostic test and associated finding?
- A. Abdominal ultrasound; elongated pyloric channel and muscle hypertrophy (Correct Answer)
- B. Barium upper GI series; GE junction and portion of the stomach in thorax
- C. Air enema; filling defect and coil spring sign
- D. Barium upper GI series; bird beak sign and corkscrewing
- E. Abdominal X-ray; ‘double bubble’ sign
Pancreas development Explanation: ***Abdominal ultrasound; elongated pyloric channel and muscle hypertrophy***
- The clinical picture of **projectile vomiting** in a 4-week-old infant, **eagerness to feed** ("hungry vomiter"), and **palpable olive-shaped mass** in the right upper quadrant is classic for **pyloric stenosis**.
- **Abdominal ultrasonography** is the gold standard for diagnosis, revealing an **elongated pyloric channel** (>16mm) and thickened pyloric muscle (>3-4mm).
- Pyloric stenosis typically presents between 3-6 weeks of age with progressive non-bilious vomiting.
*Barium upper GI series; GE junction and portion of the stomach in thorax*
- A **barium upper GI series** showing the **GE junction and stomach in the thorax** would indicate a **hiatal hernia**, which is not consistent with the palpable mass or "hungry vomiter" presentation.
- While hiatal hernias can cause vomiting and reflux, they typically don't present with this specific type of projectile vomiting or a palpable abdominal mass.
*Air enema; filling defect and coil spring sign*
- An **air enema** showing a **filling defect** and **coil spring sign** is characteristic of **intussusception**, which usually presents with sudden onset of **crampy abdominal pain**, **currant jelly stools**, and a palpable mass in the right lower quadrant.
- The clinical presentation does not fit intussusception, which typically occurs in older infants (6-36 months) and has a more acute presentation.
*Barium upper GI series; bird beak sign and corkscrewing*
- A **barium upper GI series** showing a **bird beak sign** and **corkscrewing** is pathognomonic for **midgut volvulus**, a surgical emergency.
- While volvulus can cause bilious vomiting and abdominal distension, the presentation of non-bilious vomiting with a palpable pyloric mass is more typical of pyloric stenosis.
*Abdominal X-ray; 'double bubble' sign*
- An **abdominal X-ray** revealing a **'double bubble' sign** is indicative of **duodenal atresia** or **annular pancreas**, leading to complete duodenal obstruction.
- This condition typically presents with **bilious vomiting** shortly after birth (within first day of life) and does not involve a palpable hypertrophied pylorus.
Pancreas development US Medical PG Question 3: A 43-year-old man visits his physician’s office for a routine check-up. He tells his physician that he is otherwise healthy, except for persistent headaches that he gets every morning. Upon further questioning, he reveals that he has been changing glove sizes quite frequently over the past couple of years. His wedding ring doesn’t fit him anymore. He thought this was probably due to some extra weight that he has put on. Vital signs include: blood pressure 160/90 mm Hg, heart rate 82/min, and respiratory rate 21/min. His current physical appearance is cataloged in the image. His past medical history is significant for diabetes for which he has been receiving treatment for the past 2 years. Which of the following organs most likely has a structural abnormality that has resulted in this patient’s current presentation?
- A. Liver
- B. Pancreas
- C. Posterior pituitary gland
- D. Lungs
- E. Anterior pituitary gland (Correct Answer)
Pancreas development Explanation: ***Anterior pituitary gland***
- The patient's symptoms, including **persistent headaches**, **increasing glove and ring sizes**, and **physical appearance** suggestive of facial and acral growth, are classic signs of **acromegaly**.
- **Acromegaly** is most commonly caused by a **growth hormone-secreting adenoma** of the **anterior pituitary gland**, leading to excess growth hormone production.
*Liver*
- While the liver plays a role in metabolism and produces **insulin-like growth factor 1 (IGF-1)**, it is not the primary site of pathology in acromegaly.
- Liver abnormalities would typically present with symptoms such as jaundice, fatigue, or abdominal pain, which are not the patient's primary complaints.
*Pancreas*
- The pancreas is responsible for insulin production, and its dysfunction leads to **diabetes mellitus**. While the patient has diabetes, this is often a **secondary complication** of acromegaly due to insulin resistance, rather than the primary cause of the growth-related symptoms.
- A primary pancreatic structural abnormality would not explain the generalized growth of extremities and facial features.
*Posterior pituitary gland*
- The **posterior pituitary gland** primarily secretes **vasopressin (ADH)** and **oxytocin**.
- Structural abnormalities here would typically result in disorders like **diabetes insipidus** (due to ADH deficiency) or syndromes related to oxytocin, not the growth-related symptoms seen in this patient.
*Lungs*
- Lung abnormalities can lead to various respiratory symptoms, such as shortness of breath, cough, or chest pain.
- There is no direct link between a primary structural abnormality of the lungs and the systemic growth changes or persistent headaches described in this patient.
Pancreas development US Medical PG Question 4: A 37-year-old man presents with dull, continuous epigastric pain that radiates to the back in a circumscribing fashion. The history is significant for 3 episodes of acute pancreatitis that were managed conservatively. He reports no history of such episodes in his relatives and denies a family history of any cardiovascular or gastrointestinal disorders. The vital signs include: blood pressure 105/70 mm Hg, heart rate 101/min, respiratory rate 17/min, and temperature 37.4℃ (99.3℉). The physical examination reveals epigastric tenderness, slight muscle guarding, a positive Mayo-Robson’s sign, and abdominal distention. Laboratory studies show the following findings:
Complete blood count
Erythrocytes 4.5 x 106/mm3
Hgb 14.7 g/dL
Hct 43%
Leukocytes 12,700/mm3
Segmented neutrophils 65%
Bands 4%
Eosinophils 1%
Basophils 0%
Lymphocytes 27%
Monocytes 3%
Biochemistry
Serum amylase 170 U/L
ALT 21 U/L
AST 19 U/L
Total serum cholesterol 139 mg/dL (3.6 mmol/L)
Serum triglycerides 127 mg/dL (1.4 mmol/L)
The magnetic resonance cholangiopancreatography findings are shown in the exhibit. What embryogenic disruption could cause such anatomic findings?
- A. Duplication of the pancreatic bud of the midgut
- B. Failure of fusion of dorsal and ventral pancreatic duct anlages (Correct Answer)
- C. Ectopy of the developing bile duct
- D. Duplication of the embryonic pancreatic duct
- E. Improper rotation of the ventral pancreatic bud
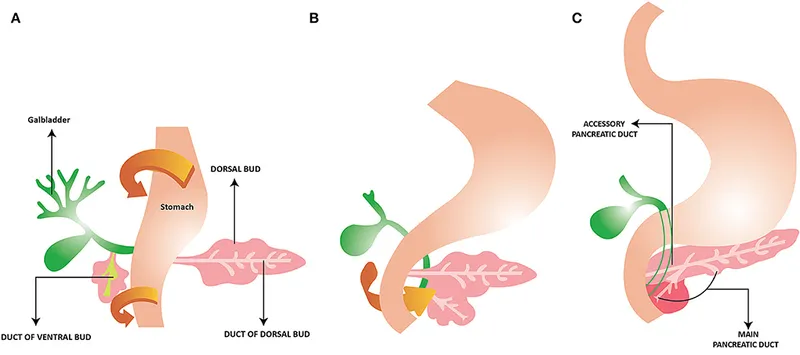
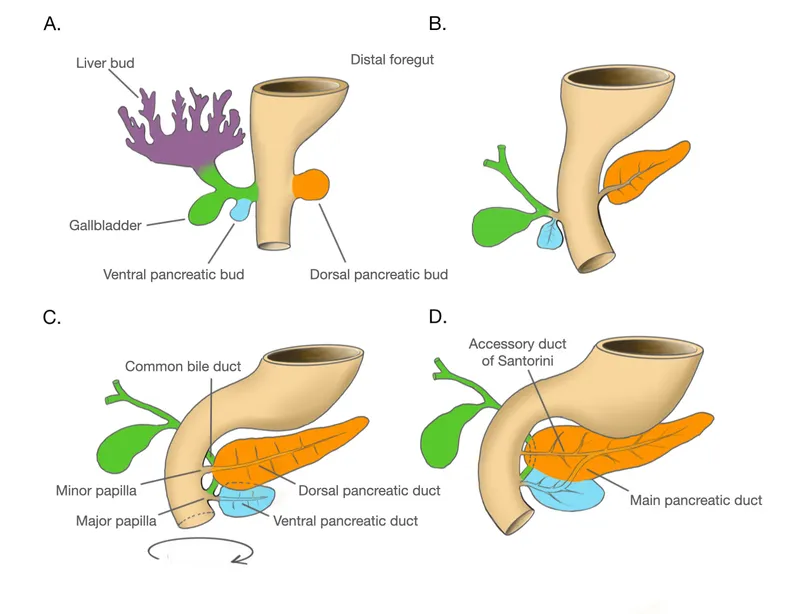
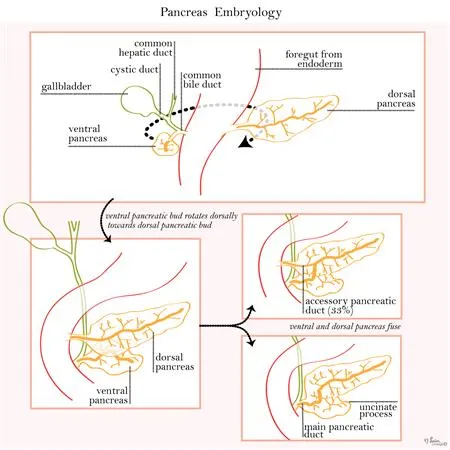
Pancreas development Explanation: ***Failure of fusion of dorsal and ventral pancreatic duct anlages***
- The patient's history of **recurrent pancreatitis**, dull epigastric pain radiating to the back, and the provided magnetic resonance cholangiopancreatography (MRCP) findings are highly suggestive of **pancreas divisum**. Pancreas divisum results from the **failure of the dorsal and ventral pancreatic ducts to fuse** during embryological development.
- In pancreas divisum, the majority of the pancreatic drainage occurs through the **minor papilla** via the dorsal pancreatic duct, which can become stenotic or obstructed, leading to increased pressure in the dorsal duct and recurrent pancreatitis.
*Improper rotation of the anterior pancreatic bud*
- **Improper rotation of the ventral pancreatic bud** (not anterior) can lead to **annular pancreas**, where pancreatic tissue encircles the duodenum.
- While annular pancreas can cause duodenal obstruction, it does not typically present with the specific MRCP findings seen in pancreas divisum or recurrent pancreatitis as the primary symptom.
*Duplication of the pancreatic bud of the midgut*
- The pancreas develops from the **foregut**, not the midgut. Duplication of pancreatic buds is a rare condition and would likely manifest differently from the typical presentation of pancreas divisum.
- Pancreatic development involves dorsal and ventral buds, not a "midgut" bud, making this option anatomically incorrect in the context of typical pancreatic anomalies.
*Ectopy of the developing bile duct*
- **Ectopy of the bile duct** refers to the bile duct opening in an abnormal location. While bile duct anomalies can occur, they are distinct from pancreatic duct fusion abnormalities.
- This would primarily cause issues related to bile drainage, such as cholestasis or cholangitis, rather than the recurrent pancreatitis and specific ductal morphology associated with pancreas divisum.
*Duplication of the embryonic pancreatic duct*
- While congenital anomalies involving pancreatic ducts exist, **duplication of the entire embryonic pancreatic duct** is not a recognized cause of pancreas divisum.
- Pancreas divisum is specifically due to a *failure of fusion* rather than an extra ductal structure stemming from an initial duplication.
Pancreas development US Medical PG Question 5: During development, a fetus is found to have incomplete fusion of the neural tube. Which of the following structures would most likely be affected by this developmental defect?
- A. Notochord
- B. Somites
- C. Vertebral bodies
- D. Spinal cord and meninges (Correct Answer)
Pancreas development Explanation: ***Spinal cord and meninges***
- Incomplete fusion of the neural tube directly results in defects of the **neural tube closure**, which include the formation of the **spinal cord** and its protective coverings, the **meninges**. [1, 2]
- Conditions like **spina bifida** (meningocele, myelomeningocele) are direct consequences of these fusion failures, exposing or abnormally developing the spinal cord and meninges. [1, 2]
*Notochord*
- The **notochord** is a transient embryonic structure that induces the formation of the neural tube by signaling to the overlying ectoderm; it is not directly formed by the neural tube itself.
- While it plays a critical role in neural tube development, its own structural integrity is typically not primarily affected by neural tube fusion defects.
*Somites*
- **Somites** are blocks of paraxial mesoderm that differentiate into sclerotome (vertebrae and ribs), myotome (skeletal muscle), and dermatome (dermis of the skin).
- While somite development is closely coordinated with neural tube formation, incomplete neural tube fusion primarily affects the neural structures themselves, not the somites directly.
*Vertebral bodies*
- **Vertebral bodies** develop from the sclerotome portion of the somites, which migrate to surround the neural tube and notochord.
- While vertebral defects can be associated with severe neural tube defects (e.g., in spina bifida, the vertebral arches may fail to close), the primary defect of incomplete neural tube fusion directly impacts the neural tissue (spinal cord and meninges), with skeletal defects being secondary or associated. [1, 2]
Pancreas development US Medical PG Question 6: A 10-month-old infant is brought in by his parents because he is vomiting and not passing stool. His parents say he has vomited multiple times over the past couple of hours, but the most recent vomit was green. The patient has no significant past medical history. On physical examination, the patient is irritable and crying. On palpation in the periumbilical region, an abdominal mass is present. Emergency laparotomy is performed, which shows a part of the patient’s intestine folded into the section adjacent to it. Which of the following is the most likely diagnosis for this patient?
- A. Pyloric stenosis
- B. Hirschsprung’s disease
- C. Duodenal atresia
- D. Intussusception (Correct Answer)
- E. Meckel’s diverticulum
Pancreas development Explanation: ***Intussusception***
- This diagnosis is highly suggested by the classic presentation of a 10-month-old infant with **bilious vomiting**, **abdominal pain** (irritability), and an **abdominal mass** in the periumbilical region, coupled with the surgical finding of one part of the intestine telescoping into an adjacent section.
- The sudden onset of symptoms in an otherwise healthy infant, along with **green vomit** (indicating bile) and an acute abdomen, are hallmark signs of this condition.
*Pyloric stenosis*
- This condition typically presents with **non-bilious projectile vomiting** in infants usually between 3 weeks and 6 months of age, with an **olive-shaped mass** in the epigastrium.
- The patient's **bilious vomiting** and the specific finding of intestinal telescoping rule out pyloric stenosis.
*Hirschsprung’s disease*
- This condition usually presents with **failure to pass meconium** in the neonatal period or chronic constipation and abdominal distension in older infants.
- While it involves the intestine, it is a **motility disorder** due to the absence of ganglion cells, not an anatomical telescoping of bowel.
*Duodenal atresia*
- This is a congenital obstruction of the duodenum, typically diagnosed shortly after birth with **bilious vomiting** and a characteristic "**double bubble**" sign on X-ray.
- It would not involve an abdominal mass or the intussusception described.
*Meckel’s diverticulum*
- This condition is a remnant of the vitelline duct and can present with painless rectal bleeding or, less commonly, intestinal obstruction, **volvulus**, or **intussusception** if it acts as a lead point.
- While it can be a rare cause of intussusception, the question directly describes the pathophysiology of intussusception itself rather than a diverticulum causing it.
Pancreas development US Medical PG Question 7: A 2-week-old boy has developed bilious vomiting. He was born via cesarean section at term. On physical exam, his pulse is 140, blood pressure is 80/50 mmHg, and respirations are 40/min. His abdomen appears distended and appears diffusely tender to palpation. Abdominal imaging is obtained (Figures A). Which of the following describes the mechanism that caused this child's disorder?
- A. Hypertrophy of the pylorus
- B. Abnormal rotation of the midgut (Correct Answer)
- C. Ischemia-reperfusion injury in premature neonate
- D. Partial absence of ganglion cells in large intestine
- E. Telescoping segment of bowel
Pancreas development Explanation: ***Abnormal rotation of the midgut***
- The presentation of **bilious vomiting** in a neonate, along with abdominal distension and tenderness, is highly suggestive of a **malrotation with midgut volvulus**. This condition results from an incomplete or abnormal rotation of the fetal midgut during development, leading to an improperly fixed mesentery.
- The narrow mesenteric base, characteristic of malrotation, predisposes to twisting of the bowel around the superior mesenteric artery, causing **bowel obstruction and ischemia**. The provided image would likely show findings consistent with obstruction, such as dilated loops of bowel and possibly signs of compromised blood flow (e.g., pneumatosis intestinalis in severe cases, though not explicitly mentioned for this image).
*Hypertrophy of the pylorus*
- This condition is known as **pyloric stenosis** and typically presents with **non-bilious projectile vomiting** around 3-6 weeks of age, not bilious vomiting.
- While it causes gastric outlet obstruction, the vomiting is non-bilious because the obstruction is proximal to the entry of bile ducts into the duodenum.
*Ischemia-reperfusion injury in premature neonate*
- This mechanism is associated with **necrotizing enterocolitis (NEC)**, which primarily affects **premature neonates** and presents with abdominal distension, feeding intolerance, and bloody stools.
- The patient in the question is a **term neonate**, making NEC less likely, and the primary symptom is bilious vomiting rather than bloody stools.
*Partial absence of ganglion cells in large intestine*
- This describes **Hirschsprung disease**, which typically presents with **constipation**, abdominal distension, and failure to pass meconium, rather than acute bilious vomiting as the primary symptom in an infant.
- While it can cause bowel obstruction, the pathology involves the large intestine and often has a more chronic presentation of obstructive symptoms.
*Telescoping segment of bowel*
- This describes **intussusception**, which is characterized by sudden onset of **intermittent, crampy abdominal pain**, **vomiting (often bilious)**, and "currant jelly" stools.
- While intussusception can cause bilious vomiting and bowel obstruction, it typically occurs in infants aged 6 months to 3 years and is less common in a 2-week-old neonate where malrotation/volvulus is more prominent for acute obstructive symptoms.
Pancreas development US Medical PG Question 8: A 60-year-old gentleman passes away after a car accident. On routine autopsy it is incidentally noted that he has both a ventral and dorsal pancreatic duct. This incidental finding observed by the pathologist is generated due to failure of which of the following embryological processes?
- A. Apoptosis
- B. Stem cell differentiation
- C. Notochord signaling
- D. Neural crest cell migration
- E. Fusion (Correct Answer)
Pancreas development Explanation: ***Fusion***
- The pancreas develops from a **ventral and a dorsal bud** that typically **fuse** during development.
- Failure of these two pancreatic buds (and their associated ducts) to completely fuse can result in **pancreas divisum**, where two separate ductal systems persist, corresponding to the dorsal and ventral pancreatic ducts.
*Apoptosis*
- **Apoptosis** (programmed cell death) is crucial for the removal of unwanted cells and sculpting tissues during embryogenesis, such as the formation of digits or the regression of certain structures.
- It does not directly explain the persistence of two separate pancreatic ducts due to non-fusion of developmental buds.
*Stem cell differentiation*
- **Stem cell differentiation** is the process by which less specialized stem cells become more specialized cell types, which is fundamental to organ development and tissue formation.
- While essential for pancreatic development, it doesn't specifically account for the anatomical anomaly of two persistent ducts.
*Notochord signaling*
- **Notochord signaling** is vital for inducing the formation of the neural tube and defining the dorsal-ventral axis of the embryo, as well as influencing the development of other nearby structures.
- This process is not directly related to the fusion of pancreatic buds, which occurs later and is influenced by interactions between mesenchymal and endodermal tissues.
*Neural crest cell migration*
- **Neural crest cells** are multipotent cells that migrate extensively throughout the embryo to form a wide variety of tissues, including parts of the peripheral nervous system, melanocytes, and bone/cartilage of the face and skull.
- Their migratory pathways and derivatives are not directly involved in the development and fusion of the pancreatic ductal system.
Pancreas development US Medical PG Question 9: A 55-year-old man comes to the physician because of a 3-week history of intermittent burning epigastric pain. His pain improves with antacid use and eating but returns approximately 2 hours following meals. He has a history of chronic osteoarthritis and takes ibuprofen daily. Upper endoscopy shows a deep ulcer located on the posterior wall of the duodenal bulb. This ulcer is most likely to erode into which of the following structures?
- A. Splenic vein
- B. Descending aorta
- C. Pancreatic duct
- D. Gastroduodenal artery (Correct Answer)
- E. Transverse colon
Pancreas development Explanation: ***Gastroduodenal artery***
- A deep ulcer on the **posterior wall of the duodenal bulb** is anatomically very close to the **gastroduodenal artery**.
- Erosion into this artery can lead to **life-threatening upper gastrointestinal bleeding**, a severe complication of peptic ulcer disease.
*Splenic vein*
- The **splenic vein** is located more posteriorly and superiorly, primarily in relation to the pancreas and spleen, making it less likely to be eroded by a duodenal bulb ulcer.
- While erosion into major vessels can occur, the gastroduodenal artery is in a much more direct and immediate proximity to the posterior duodenal bulb.
*Descending aorta*
- The **descending aorta** is a retroperitoneal structure located much more posteriorly and medially, far from the duodenal bulb.
- Erosion into the aorta is an extremely rare and catastrophic event, not typically associated with duodenal ulcers.
*Pancreatic duct*
- The **pancreatic duct** (Wirsung's duct) is located within the pancreas, which lies posterior to the duodenum. While a *deep* ulcer could hypothetically penetrate the pancreas, the primary structure at risk for hemorrhage from a posterior duodenal bulb ulcer is the gastroduodenal artery.
- Erosion into the pancreatic duct would likely cause **pancreatitis** or **fistula formation**, rather than acute hemorrhage.
*Transverse colon*
- The **transverse colon** is located inferior to the duodenum, separated by the greater omentum.
- Ulcers would typically erode anteriorly or directly posteriorly, not inferiorly into the transverse colon, which would involve fistula formation rather than arterial erosion.
Pancreas development US Medical PG Question 10: A 4-week-old infant presents with progressively worsening jaundice. Laboratory studies show direct hyperbilirubinemia, elevated gamma-glutamyl transferase, and pale stools. Liver biopsy shows bile duct proliferation and portal fibrosis. Intraoperative cholangiogram reveals absence of extrahepatic bile ducts with normal intrahepatic ducts proximally. The gallbladder is present but atretic. Synthesize the embryological timing and pathophysiological mechanism of this postnatal progressive condition.
- A. Failure of recanalization of solid bile duct stage at week 6-7 with progressive inflammation
- B. Defective hepatic diverticulum formation from foregut at week 4 causing ductal absence
- C. Perinatal inflammatory obliterative cholangiopathy affecting extrahepatic ducts after normal formation (Correct Answer)
- D. Abnormal neural crest cell migration affecting bile duct innervation and motility
- E. Congenital cytomegalovirus causing intrauterine ductal destruction at week 12
Pancreas development Explanation: ***Perinatal inflammatory obliterative cholangiopathy affecting extrahepatic ducts after normal formation***
- **Biliary atresia** is currently understood as a progressive, **acquired inflammatory** process that obliterates ducts that were initially formed correctly during embryogenesis.
- The clinical presentation of **progressive jaundice**, **pale (acholic) stools**, and **direct hyperbilirubinemia** appearing after birth supports a perinatal insult rather than an embryonic malformation.
*Failure of recanalization of solid bile duct stage at week 6-7 with progressive inflammation*
- This describes the **"tandem" theory** or failure of the solid stage, which typically results in more severe embryonic-type atresia often associated with other **congenital anomalies** (situs inversus).
- Most cases of biliary atresia do not show a failure of recanalization, as clinical evidence suggests the ducts were functional for a period in utero.
*Defective hepatic diverticulum formation from foregut at week 4 causing ductal absence*
- A defect in the **hepatic diverticulum** would result in total **agenesis** of the biliary system and potentially the liver parenchyma, not a localized postnatal obstruction.
- In this case, the **intrahepatic ducts** are normal and the gallbladder is present, which contradicts a primary defect in early foregut budding.
*Abnormal neural crest cell migration affecting bile duct innervation and motility*
- **Neural crest cell** migration defects are classically associated with **Hirschsprung disease** (affecting the enteric nervous system), not the structural obliteration of bile ducts.
- Biliary atresia involves mechanical **fibrosis and obstruction** of the lumen, rather than a primary disorder of biliary tree motility or innervation.
*Congenital cytomegalovirus causing intrauterine ductal destruction at week 12*
- While some viral triggers (like **Reovirus** or Rotavirus) are suspected in the inflammatory process, **Cytomegalovirus (CMV)** typically causes a neonatal hepatitis rather than isolated extrahepatic ductal obliteration.
- Intrauterine destruction at week 12 would likely present with symptoms immediately at birth, whereas this infant showed **progressive worsening** over 4 weeks.
More Pancreas development US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.